Optimizing targeted drug selection in combination therapy for patients with advanced or metastatic renal cell carcinoma: A systematic review and network meta-analysis of safety
- PMID: 36457303
- PMCID: PMC10067120
- DOI: 10.1002/cam4.5504
Optimizing targeted drug selection in combination therapy for patients with advanced or metastatic renal cell carcinoma: A systematic review and network meta-analysis of safety
Abstract
Objective: For patients with advanced or metastatic renal cell carcinoma (RCC), the dose of targeted agents was recommended in combination with immune checkpoint inhibitors. We performed a network meta-analysis to describe a categorized safety ranking profile and assess the adaptability of the combination options of targeted agents.
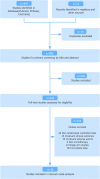
Methods: The targeted agents refer to vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) and mammalian target of rapamycin (mTOR) inhibitors. Randomized controlled trials comparing these drugs were enrolled in a Bayesian model network meta-analysis.
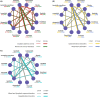
Results: Nineteen clinical trials with 11 treatments and 10,615 patients were included. For grade ≥ 3 adverse events (AEs), compared with placebo, lenvatinib plus everolimus showed worse safety than all other treatments except for lenvatinib (placebo vs. OR 0.23, 95% CI 0.07-0.78). Everolimus was generally the safest agent (OR 1.23, 95% CI 0.50-3.14). Sorafenib arose the least renal AEs (placebo vs. OR 0.85, 95% CI 0.06-11.64), whereas lenvatinib plus everolimus had the highest risk of renal toxicity (placebo vs. 0.17 95% CI 0.01-1.02). For gastrointestinal symptoms, everolimus was related to much lower toxicity than other agents. In the respiratory safety analysis, tivozanib (placebo vs. OR 0.15, 95% CI 0.07-0.31) and axitinib (OR 5.43, 95% CI 3.26-9.22) were the riskiest agents. In terms of hepatobiliary (placebo vs. OR 0.44, 95% CI 0.09-2.10) and hemotoxicity (placebo vs. OR 1.03, 95% CI 0.14-7.68) related AEs, lenvatinib was found to be the safest treatment compared to placebo.
Conclusions: Everolimus, with the best safety of grade ≥ 3, gastrointestinal, and respiratory AEs, was more likely to be considered for combination therapies. Lenvatinib appears to be the safest for blood/lymphatic and hepatobiliary AEs. For patients with renal disorders, sorafenib arises the least renal toxicity AEs. This study will guide treatment options and optimize the trial design for advanced or metastatic RCC.
Keywords: VEGF-TKI; mTOR inhibitor; renal cell carcinoma; targeted agents.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


References
-
- Tegos T, Tegos K, Dimitriadou A, Dimitriadis G. Current and emerging first‐line systemic therapies in metastatic clear‐cell renal cell carcinoma. J BUON. 2019;24(4):1340‐1353. https://www.ncbi.nlm.nih.gov/pubmed/31646776 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

