Functional tuning of Vascular L-type Ca2+ channels
- PMID: 36457306
- PMCID: PMC9705771
- DOI: 10.3389/fphys.2022.1058744
Functional tuning of Vascular L-type Ca2+ channels
Abstract
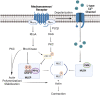
Vascular smooth muscle contraction is intimately tied to membrane potential and the rise in intracellular Ca2+ enabled by the opening of L-type Ca2+ channels. While voltage is often viewed as the single critical factor gating these channels, research is starting to reveal a more intricate scenario whereby their function is markedly tuned. This emerging concept will be the focus of this three-part review, the first part articulating the mechanistic foundation of contractile development in vascular smooth muscle. Part two will extend this foundational knowledge, introducing readers to functional coupling and how neighboring L-type Ca2+ channels work cooperatively through signaling protein complexes, to facilitate their open probability. The final aspect of this review will discuss the impact of L-type Ca2+ channel trafficking, a process tied to cytoskeleton dynamics. Cumulatively, this brief manuscript provides new insight into how voltage, along with channel cooperativity and number, work in concert to tune Ca2+ responses and smooth muscle contraction.
Keywords: Ca2+ sensitization; L-type Ca2+ channels; channel trafficking; cooperative gating; functional coupling; vascular smooth muscle.
Copyright © 2022 Mironova, Haghbin and Welsh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[Ca2+]i inhibition of K+ channels in canine renal artery. Novel mechanism for agonist-induced membrane depolarization.Circ Res. 1995 Jul;77(1):121-30. doi: 10.1161/01.res.77.1.121. Circ Res. 1995. Retraction in: Circ Res. 2004 Jan 9;94(1):e15. doi: 10.1161/01.RES.0000113471.89285.E3. PMID: 7788870 Retracted.
-
Coupling and cooperativity in voltage activation of a limited-state BK channel gating in saturating Ca2+.J Gen Physiol. 2010 May;135(5):461-80. doi: 10.1085/jgp.200910331. J Gen Physiol. 2010. PMID: 20421372 Free PMC article.
-
β-adrenergic-mediated dynamic augmentation of sarcolemmal CaV 1.2 clustering and co-operativity in ventricular myocytes.J Physiol. 2019 Apr;597(8):2139-2162. doi: 10.1113/JP277283. Epub 2019 Mar 12. J Physiol. 2019. PMID: 30714156 Free PMC article.
-
Local regulation of L-type Ca²⁺ channel sparklets in arterial smooth muscle.Microcirculation. 2013 May;20(4):290-8. doi: 10.1111/micc.12021. Microcirculation. 2013. PMID: 23116449 Free PMC article. Review.
-
Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone.Acta Physiol Scand. 1998 Dec;164(4):577-87. doi: 10.1046/j.1365-201X.1998.00462.x. Acta Physiol Scand. 1998. PMID: 9887980 Review.
Cited by
-
The role and mechanism of vascular wall cell ion channels in vascular fibrosis remodeling.Channels (Austin). 2024 Dec;18(1):2418128. doi: 10.1080/19336950.2024.2418128. Epub 2024 Oct 19. Channels (Austin). 2024. PMID: 39425532 Free PMC article. Review.
-
T-Type Voltage-Gated Calcium Channels: Potential Regulators of Smooth Muscle Contractility.Int J Mol Sci. 2024 Nov 19;25(22):12420. doi: 10.3390/ijms252212420. Int J Mol Sci. 2024. PMID: 39596484 Free PMC article. Review.
References
-
- Amberg G. C., Navedo M. F., Nieves-cintrón M., Molkentin J. D., Santana L. F. (2007). Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J. Physiol. 579 (1), 187–201. accessed September 21, 2022, from/pmc/articles/PMC2075382/, February 2. 10.1113/JPHYSIOL.2006.124420 - DOI - PMC - PubMed
-
- Bannister J. P., Bulley S., Narayanan D., Thomas-Gatewood C., Luzny P., Pachuau J., et al. (1979). Transcriptional upregulation of α2δ-1 elevates arterial smooth muscle cell voltage-dependent Ca2+ channel surface expression and cerebrovascular constriction in genetic hypertension. Hypertension 60 (4), 1006–1015. accessed September 25, 2022, from https://pubmed.ncbi.nlm.nih.gov/22949532/, October 2012. 10.1161/HYPERTENSIONAHA.112.199661 - DOI - PMC - PubMed
-
- Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., et al. (1995). Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Sci. (New York, N.Y.) 267 (5194), 108–111. accessed September 21, 2022, from https://pubmed.ncbi.nlm.nih.gov/7528941/. 10.1126/SCIENCE.7528941 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

