Effectiveness of Abdominal Functional Electrical Stimulation for Improving Bowel Function in People With a Spinal Cord Injury: A Study Protocol for a Double-Blinded Randomized Placebo-Controlled Clinical Trial
- PMID: 36457354
- PMCID: PMC9678222
- DOI: 10.46292/sci22-00008
Effectiveness of Abdominal Functional Electrical Stimulation for Improving Bowel Function in People With a Spinal Cord Injury: A Study Protocol for a Double-Blinded Randomized Placebo-Controlled Clinical Trial
Abstract
Background: People with a spinal cord injury (SCI) have a high rate of bowel-related morbidity, even compared with people with other neurological disorders. These complications lower quality of life and place a financial burden on the health system. A noninvasive intervention that improves the bowel function of people with an SCI should reduce morbidity, improve quality of life, and lead to cost savings for health care providers.
Objectives: To investigate the effectiveness of noninvasive abdominal functional electrical stimulation (FES) for improving bowel function in people with a chronic SCI.
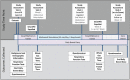
Methods: A prospective, double-blinded, 1:1 randomized, placebo-controlled intervention trial will be conducted with 80 adults with chronic SCI (>12 months since injury) above T8 single neurological level. The intervention will be a 45-minute abdominal FES (or placebo) session, 3 days per week, for 6 weeks.
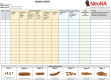
Main study parameters/endpoints: Primary endpoint is whole gut transit time before and after 6 weeks of abdominal FES. Secondary endpoints measured before and after 6 weeks of abdominal FES are (1) colonic transit time; (2) quality of life (EQ-5D-5L); (3) participant-reported bowel function (International SCI Bowel Function Basic Data Set Questionnaire and visual analogue scale); (4) respiratory function (forced vital capacity, forced expiratory volume in 1 second, peak expiratory flow, maximal inspiratory pressure, and maximal expiratory pressure); (5) bladder symptoms (Neurogenic Bladder Symptom Score); (6) daily bowel management diary; and (7) unplanned hospital visits.
Conclusion: Safety data will be collected, and a cost utility analysis using quality of life scores will be performed.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12621000386831.
Keywords: abdominal functional electrical stimulation; bowel function; respiratory function; spinal cord injury; whole gut transit time.
© 2022 American Spinal Injury Association.
Conflict of interest statement
Conflicts of Interest The authors declare no conflicts of interest.
Figures
References
-
- Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther . 2002;82(6):601–612. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous