Efficacy and safety of belantamab-mafodotin in triple-refractory multiple myeloma patients: A multicentric real-life experience
- PMID: 36457484
- PMCID: PMC9705330
- DOI: 10.3389/fonc.2022.1026251
Efficacy and safety of belantamab-mafodotin in triple-refractory multiple myeloma patients: A multicentric real-life experience
Abstract
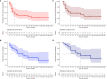
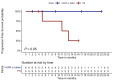
Belantamab-mafodotin is an innovative and selective treatment for multi-refractory/relapsed multiple myeloma (MM) patients; however, available real-life experiences on efficacy and safety are limited. In this real-world multicentric retrospective study, we enrolled 28 MM patients treated in four Hematology units of Campania region, Italy, who received a median of six treatment lines prior to belantamab-mafodotin. The overall response rate (ORR) was 40% (complete remission, CR, 11%; very good partial remission, VGPR, 11%; and partial remission, PR, 18%), with a median progression-free survival (PFS) and overall survival (OS) of 3 and 8 months, respectively. One of the most frequent drug-related adverse events was keratopathy observed in nine (32%) patients, leading to therapy discontinuation in only three (11%) of them. Moreover, 22 out of 28 total patients who were treated with at least two administrations achieved an ORR of 50% (CR, 14%; VGPR, 14%; and PR, 22%) with a median PFS and OS of 5 and 11 months, respectively. In conclusion, our multicentric study confirmed efficacy and safety of belantamab-mafodotin in triple-refractory MM patients even in the real-life setting.
Keywords: anti-BCMA; multi-refractory; multiple myeloma; real life; refractory.
Copyright © 2022 Iula, De Novellis, Trastulli, Della Pepa, Fontana, Carobene, Di Perna, D’Ambrosio, Romano, Leone, De Fazio, Fiumarella, Gaeta, Marafioti, Barbato, Palmieri, Rocco, Serio, Califano, Pane, Ferrara, Giudice, Selleri and Catalano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

