HAT-field: a cheap, robust and quantitative Point-of-care serological test for Covid-19
- PMID: 36457547
- PMCID: PMC9620368
- DOI: 10.1093/biomethods/bpac026
HAT-field: a cheap, robust and quantitative Point-of-care serological test for Covid-19
Abstract
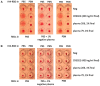
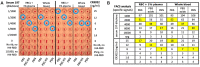
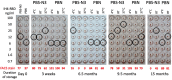
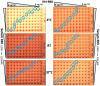
The haemagglutination test (HAT)-field protocol described here is an optimization of the recently published HAT, for the detection of antibodies directed against the receptor binding domain (RBD) of the SARS-Cov-2 virus. HAT and HAT-field are both based on haemagglutination triggered by a single reagent, the IH4-RBD recombinant protein. A sample of IH4-RBD sufficient for several thousand tests or a plasmid encoding IH4-RBD can be obtained from the authors of our first paper. Using titration of IH4-RBD, HAT-field now allows a quantitative assessment of antibody levels in a single step, using a few microliters of whole blood, such as can be obtained by finger prick, and requires only very simple disposable equipment. Because it is based on a single soluble reagent, the test can be adapted very simply and rapidly to detect antibodies against variants of the SARS-CoV-2, or conceivably against different pathogens. HAT-field appears well suited to provide quantitative assessments of the serological protection of populations as well as individuals, and given its very low cost, the stability of the IH4-RBD reagent in the adapted buffer and the simplicity of the procedure, could be deployed pretty much anywhere, including in the poorest countries and the most remote corners of the globe.
Keywords: Covid-19; IH4-RBD; SARS-Cov-2; haemagglutination; quantitative.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
