Crucial role of T cells in NAFLD-related disease: A review and prospect
- PMID: 36457551
- PMCID: PMC9705593
- DOI: 10.3389/fendo.2022.1051076
Crucial role of T cells in NAFLD-related disease: A review and prospect
Abstract
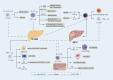
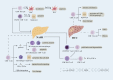
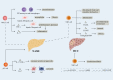
Nonalcoholic fatty liver disease (NAFLD) includes a series of hepatic manifestations, starting with liver steatosis and potentially evolving towards nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis or even hepatocellular carcinoma (HCC). Its incidence is increasing worldwide. Several factors including metabolic dysfunction, oxidative stress, lipotoxicity contribute to the liver inflammation. Several immune cell-mediated inflammatory processes are involved in NAFLD in which T cells play a crucial part in the progression of the disease. In this review, we focus on the role of different subsets of both conventional and unconventional T cells in pathogenesis of NAFLD. Factors regarding inflammation and potential therapeutic approaches targeting immune cells in NASH are also discussed.
Keywords: CD4+ T cells; CD8+ T cells; hepatocellular carcinoma (HCC); nonalcoholic fatty liver disease (NAFLD); nonalcoholic steatohepatitis (NASH).
Copyright © 2022 Mao, Yang, Luo and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma.Front Endocrinol (Lausanne). 2021 Oct 28;12:760860. doi: 10.3389/fendo.2021.760860. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34777255 Free PMC article. Review.
-
Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Gastroenterol. 2014 Nov 14;20(42):15539-48. doi: 10.3748/wjg.v20.i42.15539. World J Gastroenterol. 2014. PMID: 25400438 Free PMC article. Review.
-
Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits.J Hepatol. 2022 Oct;77(4):1136-1160. doi: 10.1016/j.jhep.2022.06.012. Epub 2022 Jun 22. J Hepatol. 2022. PMID: 35750137 Review.
-
Utilization of animal models to investigate nonalcoholic steatohepatitis-associated hepatocellular carcinoma.Oncotarget. 2016 Jul 5;7(27):42762-42776. doi: 10.18632/oncotarget.8641. Oncotarget. 2016. PMID: 27072576 Free PMC article. Review.
-
Pattern recognition receptors in the development of nonalcoholic fatty liver disease and progression to hepatocellular carcinoma: An emerging therapeutic strategy.Front Endocrinol (Lausanne). 2023 Mar 20;14:1145392. doi: 10.3389/fendo.2023.1145392. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37020586 Free PMC article. Review.
Cited by
-
Multiscale integrative analyses unveil immune-related diagnostic signature for the progression of MASLD.Hepatol Commun. 2023 Oct 18;7(11):e0298. doi: 10.1097/HC9.0000000000000298. eCollection 2023 Nov 1. Hepatol Commun. 2023. PMID: 37851406 Free PMC article.
-
Role of gut microbiota and immune cells in metabolic-associated fatty liver disease: clinical impact.Hepatol Int. 2024 Oct;18(Suppl 2):861-872. doi: 10.1007/s12072-024-10674-6. Epub 2024 Jul 12. Hepatol Int. 2024. PMID: 38995341 Review.
-
Expression of immune related genes and possible regulatory mechanisms in different stages of non-alcoholic fatty liver disease.Front Immunol. 2024 Mar 8;15:1364442. doi: 10.3389/fimmu.2024.1364442. eCollection 2024. Front Immunol. 2024. PMID: 38524129 Free PMC article.
-
Immune system dysregulation in the pathogenesis of non-alcoholic steatohepatitis: unveiling the critical role of T and B lymphocytes.Front Immunol. 2024 Aug 1;15:1445634. doi: 10.3389/fimmu.2024.1445634. eCollection 2024. Front Immunol. 2024. PMID: 39148730 Free PMC article. Review.
-
The PNPLA3 I148M variant is associated with immune cell infiltration and advanced fibrosis in MASLD: a prospective genotype-phenotype study.J Gastroenterol. 2025 Jul 21. doi: 10.1007/s00535-025-02285-1. Online ahead of print. J Gastroenterol. 2025. PMID: 40691355
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

