Development and external validation of a novel nomogram model for predicting postoperative recurrence-free survival in non-muscle-invasive bladder cancer
- PMID: 36458001
- PMCID: PMC9706099
- DOI: 10.3389/fimmu.2022.1070043
Development and external validation of a novel nomogram model for predicting postoperative recurrence-free survival in non-muscle-invasive bladder cancer
Abstract
Background: Transurethral resection of the bladder tumor with or without adjuvant intravesical instillation (IVI) has been the standard treatment for non-muscle-invasive bladder cancer (NMIBC), whereas a high percentage of patients still experience local tumor recurrence and disease progression after receiving the standard treatment modalities. Unfortunately, current relevant prediction models for determining the recurrent and progression risk of NMIBC patients are far from impeccable.
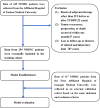
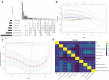
Methods: Clinicopathological characteristics and follow-up information were retrospectively collected from two tertiary medical centers between October 2018 and June 2021. The least absolute shrinkage and selection operator (LASSO) and Cox regression analysis were used to screen potential risk factors affecting recurrence-free survival (RFS) of patients. A nomogram model was established, and the patients were risk-stratified based on the model scores. Both internal and external validation were performed by sampling the model with 1,000 bootstrap resamples.
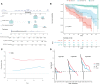
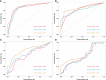
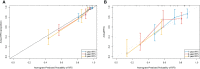
Results: The study included 299 patient data obtained from the Affiliated Hospital of Xuzhou Medical University and 117 patient data obtained from the First Affiliated Hospital of Guangxi Medical University. Univariate regression analysis suggested that urine red blood cell count and different tumor invasion locations might be potential predictors of RFS. LASSO-Cox regression confirmed that prior recurrence status, times of IVI, and systemic immune-inflammation index (SII) were independent factors for predicting RFS. The area under the curve for predicting 1-, 2-, and 3-year RFS was 0.835, 0.833, and 0.871, respectively. Based on the risk stratification, patients at high risk of recurrence and progression could be accurately identified. A user-friendly risk calculator based on the model is deposited at https://dl0710.shinyapps.io/nmibc_rfs/.
Conclusion: Internal and external validation analyses showed that our model had excellent predictive discriminatory ability and stability. The risk calculator can be used for individualized assessment of survival risk in NMIBC patients and can assist in guiding clinical decision-making.
Keywords: NMIBC; bladder cancer; nomogram; predictive indicator; risk calculator; risk factor; tumor recurrence.
Copyright © 2022 Ding, Deng, Xia, Wang, Zhang, Zhang, Shao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Development and external validation of a model to predict recurrence in patients with non-muscle invasive bladder cancer.Front Immunol. 2025 Jan 10;15:1467527. doi: 10.3389/fimmu.2024.1467527. eCollection 2024. Front Immunol. 2025. PMID: 39867903 Free PMC article.
-
Development of a dynamic risk system for predicting the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer after thulium laser resection of bladder tumor or transurethral resection of bladder tumor followed by intravesical BCG instillation.Front Oncol. 2023 Jul 5;13:1133161. doi: 10.3389/fonc.2023.1133161. eCollection 2023. Front Oncol. 2023. PMID: 37476386 Free PMC article.
-
Novel nomograms to predict recurrence and progression in primary non-muscle-invasive bladder cancer: validation of predictive efficacy in comparison with European Organization of Research and Treatment of Cancer scoring system.World J Urol. 2019 Sep;37(9):1867-1877. doi: 10.1007/s00345-018-2581-3. Epub 2018 Dec 10. World J Urol. 2019. PMID: 30535715
-
Preoperative fluorescence in situ hybridization analysis as a predictor of tumor recurrence in patients with non-muscle invasive bladder cancer: a bi-institutional study.J Transl Med. 2023 Oct 2;21(1):685. doi: 10.1186/s12967-023-04528-2. J Transl Med. 2023. PMID: 37784106 Free PMC article.
-
Predicting Recurrence and Progression in Patients with Non-Muscle-Invasive Bladder Cancer: Systematic Review on the Performance of Risk Stratification Models.Bladder Cancer. 2022 Dec 14;8(4):339-357. doi: 10.3233/BLC-220055. eCollection 2022. Bladder Cancer. 2022. PMID: 38994181 Free PMC article.
Cited by
-
Development and external validation of a model to predict recurrence in patients with non-muscle invasive bladder cancer.Front Immunol. 2025 Jan 10;15:1467527. doi: 10.3389/fimmu.2024.1467527. eCollection 2024. Front Immunol. 2025. PMID: 39867903 Free PMC article.
-
The relationship between inflammatory response markers and the prognosis of non-muscle invasive bladder cancer and the development of a nomogram model.Front Oncol. 2023 Jun 29;13:1189086. doi: 10.3389/fonc.2023.1189086. eCollection 2023. Front Oncol. 2023. PMID: 37456236 Free PMC article.
-
The prognostic impact of tumor location in nonmuscle-invasive bladder cancer patients undergoing transurethral resection: insights from a cohort study utilizing Chinese multicenter and SEER registries.Int J Surg. 2024 Sep 1;110(9):5641-5651. doi: 10.1097/JS9.0000000000001675. Int J Surg. 2024. PMID: 38788195 Free PMC article.
References
-
- Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. . Predicting recurrence and progression in individual patients with stage ta t1 bladder cancer using eortc risk tables: a combined analysis of 2596 patients from seven eortc trials. Eur Urol (2006) 49:466–77. doi: 10.1016/j.eururo.2005.12.031 - DOI - PubMed
-
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. . Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus calmette-guerin: the cueto scoring model. J Urol (2009) 182:2195–203. doi: 10.1016/j.juro.2009.07.016 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

