Myosin 1b Participated in the Modulation of Hypoxia/Reoxygenation-Caused H9c2 Cell Apoptosis and Autophagy
- PMID: 36458211
- PMCID: PMC9708368
- DOI: 10.1155/2022/5187304
Myosin 1b Participated in the Modulation of Hypoxia/Reoxygenation-Caused H9c2 Cell Apoptosis and Autophagy
Abstract
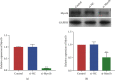
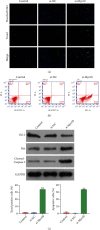
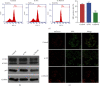
Myocardial ischemia/reperfusion (I/R) injury seriously threats the health and life of patients with ischemia heart disease. Herein, we probed the potential influence of myosin 1b (myo1b) on hypoxia/reoxygenation- (H/R-) stimulated cardiomyocyte H9c2 cell apoptosis and autophagy. After H/R stimulation, the myo1b mRNA level in H9c2 cells was tested via qRT-PCR. Myo1b overexpression plasmid (OE-myo1b) and small interfering RNA (siRNA) targeting myo1b (si-myo1b) were transfected into H9c2 cells to alter myo1b expression in H9c2 cells. Following H/R stimulation and/or OE-myo1b (or si-myo1b) transfection, H9c2 cell apoptosis, proliferation, and autophagy were detected, respectively. We found that H/R stimulation reduced the mRNA level of myo1b in H9c2 cells and resulted in H9c2 cell apoptosis, proliferation inhibition, and autophagy. Overexpression of myo1b reversed the H/R-resulted H9c2 cell apoptosis, proliferation inhibition, and autophagy. Silence of myo1b had opposite effects, which promoted H9c2 cell apoptosis, reduced cell proliferation, and accelerated cell autophagy. Taken together, Myo1b took part in the modulation of H/R-stimulated cardiomyocyte apoptosis and autophagy, which might be serve as a potential endogenous target for prevention and therapy of I/R injury.
Copyright © 2022 Jing Xu et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Epigallocatechin-3-gallate protects cardiomyocytes from hypoxia-reoxygenation damage via raising autophagy related 4C expression.Bioengineered. 2021 Dec;12(2):9496-9506. doi: 10.1080/21655979.2021.1996018. Bioengineered. 2021. PMID: 34699312 Free PMC article.
-
MicroRNA‑494 suppresses hypoxia/reoxygenation‑induced cardiomyocyte apoptosis and autophagy via the PI3K/AKT/mTOR signaling pathway by targeting SIRT1.Mol Med Rep. 2020 Dec;22(6):5231-5242. doi: 10.3892/mmr.2020.11636. Epub 2020 Oct 26. Mol Med Rep. 2020. PMID: 33174056 Free PMC article.
-
MicroRNA-489 promotes cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury through inhibiting SPIN1.Eur Rev Med Pharmacol Sci. 2019 Aug;23(15):6683-6690. doi: 10.26355/eurrev_201908_18559. Eur Rev Med Pharmacol Sci. 2019. PMID: 31378911
-
Mechanism of interactions between endoplasmic reticulum stress and autophagy in hypoxia/reoxygenation‑induced injury of H9c2 cardiomyocytes.Mol Med Rep. 2019 Jul;20(1):350-358. doi: 10.3892/mmr.2019.10228. Epub 2019 May 9. Mol Med Rep. 2019. PMID: 31115545 Free PMC article.
-
Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia.Mil Med Res. 2021 Nov 10;8(1):58. doi: 10.1186/s40779-021-00353-0. Mil Med Res. 2021. PMID: 34753510 Free PMC article.
Cited by
-
Enzyme Replacement Therapy for FABRY Disease: Possible Strategies to Improve Its Efficacy.Int J Mol Sci. 2023 Feb 25;24(5):4548. doi: 10.3390/ijms24054548. Int J Mol Sci. 2023. PMID: 36901983 Free PMC article.
-
Myo1b Promotes Premature Endothelial Senescence and Dysfunction via Suppressing Autophagy: Implications for Vascular Aging.Oxid Med Cell Longev. 2023 Jan 9;2023:4654083. doi: 10.1155/2023/4654083. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36654782 Free PMC article.
-
CKLF1, transcriptionally activated by FOXC1, promotes hypoxia/reoxygenation‑induced oxidative stress and inflammation in H9c2 cells by NLRP3 inflammasome activation.Exp Ther Med. 2023 Dec 11;27(2):59. doi: 10.3892/etm.2023.12347. eCollection 2024 Feb. Exp Ther Med. 2023. PMID: 38234613 Free PMC article.
-
Hypoxia and re-oxygenation effects on human cardiomyocytes cultured on polycaprolactone and polyurethane nanofibrous mats.J Biol Eng. 2024 Jun 6;18(1):37. doi: 10.1186/s13036-024-00432-5. J Biol Eng. 2024. PMID: 38844979 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

