Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3
- PMID: 36458260
- PMCID: PMC9707070
- DOI: 10.1016/j.isci.2022.105631
Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3
Abstract
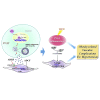
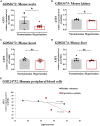
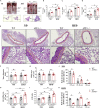
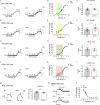
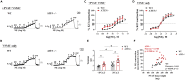
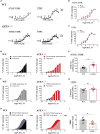
Thoracic aortic perivascular adipose tissue (PVAT) is an adipose organ exhibiting similarities to brown adipose tissue (BAT), including cellular morphology and thermogenic gene expression. However, whether the PVAT phenotype is indistinguishable from the BAT phenotype in physiological vasculature remains unclear. We demonstrated that PVAT is distinguishable from classical BAT, given its specific vessel-tone-controlling function. Activating transcription factor 3 (ATF3) is a key factor in hypertension. Compared with wild-type mice, ATF3-deficient (ATF3 -/- ) mice fed a high-fat diet exhibited elevated mean arterial pressure, increased monocyte chemoattractant protein-1 expression and hypertrophy, plus abnormal fatty tissue accumulation in the thoracic aortic PVAT, and enhanced vascular wall tension and vasoconstrictive responses of potassium chloride, U46619, and norepinephrine in isolated aortic rings, which were restored after administration of adeno-associated ATF3 vector. We suggest that PVAT, not BAT, modulates obesity-related vascular dysfunction. ATF3 within PVAT could provide new insights into the pathophysiology of obesity-related cardiovascular diseases.
Keywords: Cardiovascular medicine; Molecular biology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Activating transcription factor-3 orchestrates the modulation of vascular anti-contractile activity and relaxation by governing the secretion of HDL-bound sphingosine-1-phosphate in perivascular adipose tissue.Br J Pharmacol. 2025 Apr;182(8):1763-1782. doi: 10.1111/bph.17433. Epub 2025 Jan 22. Br J Pharmacol. 2025. PMID: 39843165
-
miRNA-22 is involved in the aortic reactivity in physiological conditions and mediates obesity-induced perivascular adipose tissue dysfunction.Life Sci. 2023 Mar 1;316:121416. doi: 10.1016/j.lfs.2023.121416. Epub 2023 Jan 21. Life Sci. 2023. PMID: 36690245
-
Dahl SS rats demonstrate enhanced aortic perivascular adipose tissue-mediated buffering of vasoconstriction through activation of NOS in the endothelium.Am J Physiol Regul Integr Comp Physiol. 2016 Feb 1;310(3):R286-96. doi: 10.1152/ajpregu.00469.2014. Epub 2015 Nov 25. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26608658 Free PMC article.
-
PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function.Front Physiol. 2018 Feb 6;9:70. doi: 10.3389/fphys.2018.00070. eCollection 2018. Front Physiol. 2018. PMID: 29467675 Free PMC article. Review.
-
Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation.Redox Biol. 2023 Jun;62:102683. doi: 10.1016/j.redox.2023.102683. Epub 2023 Mar 20. Redox Biol. 2023. PMID: 36958248 Free PMC article. Review.
Cited by
-
PVAT adipocyte: energizing, modulating, and structuring vascular function during normotensive and hypertensive states.Am J Physiol Heart Circ Physiol. 2025 Jun 1;328(6):H1204-H1217. doi: 10.1152/ajpheart.00093.2025. Epub 2025 Apr 18. Am J Physiol Heart Circ Physiol. 2025. PMID: 40250838 Free PMC article. Review.
-
Perivascular adipose tissue and adipocyte-derived exosomal miRNAs maintain vascular homeostasis.Heliyon. 2023 Nov 20;9(12):e22607. doi: 10.1016/j.heliyon.2023.e22607. eCollection 2023 Dec. Heliyon. 2023. PMID: 38076178 Free PMC article. Review.
-
The Association between Serum Adiponectin Levels and Endothelial Function in Non-Dialysis-Dependent Chronic Kidney Disease Patients.Biomedicines. 2023 Aug 2;11(8):2174. doi: 10.3390/biomedicines11082174. Biomedicines. 2023. PMID: 37626670 Free PMC article.
-
Caralluma fimbriata Extract Improves Vascular Dysfunction in Obese Mice Fed a High-Fat Diet.Nutrients. 2024 Dec 12;16(24):4296. doi: 10.3390/nu16244296. Nutrients. 2024. PMID: 39770917 Free PMC article.
-
Fat and inflammation: adipocyte-myeloid cell crosstalk in atherosclerosis.Front Immunol. 2023 Sep 15;14:1238664. doi: 10.3389/fimmu.2023.1238664. eCollection 2023. Front Immunol. 2023. PMID: 37781401 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

