Omicron-specific mRNA vaccine induced cross-protective immunity against ancestral SARS-CoV-2 infection with low neutralizing antibodies
- PMID: 36458553
- PMCID: PMC9877661
- DOI: 10.1002/jmv.28370
Omicron-specific mRNA vaccine induced cross-protective immunity against ancestral SARS-CoV-2 infection with low neutralizing antibodies
Abstract
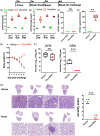
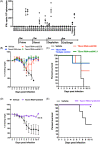
The major challenge in COVID-19 vaccine effectiveness is immune escape by SARS-CoV-2 variants. To overcome this, an Omicron-specific messenger RNA (mRNA) vaccine was designed. The extracellular domain of the spike of the Omicron variant was fused with a modified GCN4 trimerization domain with low immunogenicity (TSomi). After immunization with TSomi mRNA in hamsters, animals were challenged with SARS-CoV-2 virus. The raised nonneutralizing antibodies or cytokine secretion responses can recognize both Wuhan S and Omicron S. However, the raised antibodies neutralized SARS-CoV-2 Omicron virus infection but failed to generate Wuhan virus neutralizing antibodies. Surprisingly, TSomi mRNA immunization protected animals from Wuhan virus challenge. These data indicated that non-neutralizing antibodies or cellular immunity may play a more important role in vaccine-induced protection than previously believed. Next-generation COVID-19 vaccines using the Omicron S antigen may provide sufficient protection against ancestral or current SARS-CoV-2 variants.
Keywords: COVID-19; Omicron; SARS-CoV-2; mRNA vaccine.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Center for Systems Science and Engineering (CSSE) at Johns Hopkins University . COVID‐19 Dashboard . 2022. Accessed September 02, 2020. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

