Alarming situation of emerging H5 and H7 avian influenza and effective control strategies
- PMID: 36458831
- PMCID: PMC9754034
- DOI: 10.1080/22221751.2022.2155072
Alarming situation of emerging H5 and H7 avian influenza and effective control strategies
Abstract
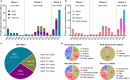
Avian influenza viruses continue to present challenges to animal and human health. Viruses bearing the hemagglutinin (HA) gene of the H5 subtype and H7 subtype have caused 2634 human cases around the world, including more than 1000 deaths. These viruses have caused numerous disease outbreaks in wild birds and domestic poultry, and are responsible for the loss of at least 422 million domestic birds since 2005. The H5 influenza viruses are spread by migratory wild birds and have caused three waves of influenza outbreaks across multiple continents, and the third wave that started in 2020 is ongoing. Many countries in Europe and North America control highly pathogenic avian influenza by culling alone, whereas some countries, including China, have adopted a "cull plus vaccination" strategy. As the largest poultry-producing country in the world, China lost relatively few poultry during the three waves of global H5 avian influenza outbreaks, and nearly eliminated the pervasive H7N9 viruses that emerged in 2013. In this review, we briefly summarize the damages the H5 and H7 influenza viruses have caused to the global poultry industry and public health, analyze the origin, evolution, and spread of the H5 viruses that caused the waves, and discuss how and why the vaccination strategy in China has been a success. Given that the H5N1 viruses are widely circulating in wild birds and causing problems in domestic poultry around the world, we recommend that any unnecessary obstacles to vaccination strategies should be removed immediately and forever.
Keywords: H5 and H7 avian influenza; Review; evolution; spread; vaccination.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Gao R, Cao B, Hu Y, et al. . Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888-1897. - PubMed
-
- Subbarao K, Klimov A, Katz J, et al. . Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–396. - PubMed
-
- Kurtz J, Manvell RJ, Banks J.. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996;348(9031):901–902. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
