A novel anti-NGF PEGylated Fab' provides analgesia with lower risk of adverse effects
- PMID: 36458900
- PMCID: PMC9721442
- DOI: 10.1080/19420862.2022.2149055
A novel anti-NGF PEGylated Fab' provides analgesia with lower risk of adverse effects
Abstract
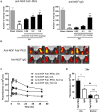
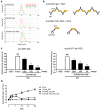
Nerve growth factor (NGF) has emerged as a key driver of pain perception in several chronic pain conditions, including osteoarthritis (OA), and plays an important role in the generation and survival of neurons. Although anti-NGF antibodies improve pain control and physical function in patients with clinical chronic pain conditions, anti-NGF IgGs are associated with safety concerns such as effects on fetal and postnatal development and the risk of rapidly progressive osteoarthritis. To overcome these drawbacks, we generated a novel anti-NGF PEGylated Fab' antibody. The anti-NGF PEGylated Fab' showed specific binding to and biological inhibitory activity against NGF, and analgesic effects in adjuvant-induced arthritis model mice in a similar manner to an anti-NGF IgG. In collagen-induced arthritis model mice, the anti-NGF PEGylated Fab' showed higher accumulation in inflamed foot pads than the anti-NGF IgG. In pregnant rats and non-human primates, the anti-NGF PEGylated Fab' was undetectable in fetuses, while the anti-NGF IgG was detected and caused abnormal postnatal development. The PEGylated Fab' and IgG also differed in their ability to form immune complexes in vitro. Additionally, while both PEGylated Fab' and IgG showed analgesic effects in sodium monoiodoacetate-induced arthritic model rats, their effects on edema were surprisingly quite different. While the anti-NGF IgG promoted edema over time, the anti-NGF PEGylated Fab' did not. The anti-NGF PEGylated Fab' (ASP6294) may thus be a potential therapeutic candidate with lower risk of adverse effects for various diseases in which NGF is involved such as OA and chronic back pain.
Keywords: NGF; PEGylated antibody; arthritis; fab; immune complex; pain; placental transfer.
Conflict of interest statement
This research was funded by Astellas Pharma. The authors are all employees of Astellas Pharma. Astellas Pharma holds patents (PCT/JP2012/070433) on the anti-human NGF antibody. Y.K., M.K, H.T., and E.Y. are inventors on the patent.
Figures


References
-
- Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the third national health and nutrition examination survey 1991-94. J Rheumatol. 2006;33(11):2271–79. PMID: 17013996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
