Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy
- PMID: 36459240
- PMCID: PMC10119234
- DOI: 10.1007/s10456-022-09863-4
Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy
Abstract
Purpose: Ongoing angiogenesis renders the tumor endothelium unresponsive to inflammatory cytokines and interferes with adhesion of leukocytes, resulting in escape from immunity. This process is referred to as tumor endothelial cell anergy. We aimed to investigate whether anti-angiogenic agents can overcome endothelial cell anergy and provide pro-inflammatory conditions.
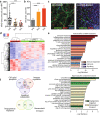
Experimental design: Tissues of renal cell carcinoma (RCC) patients treated with VEGF pathway-targeted drugs and control tissues were subject to RNAseq and immunohistochemical profiling of the leukocyte infiltrate. Analysis of adhesion molecule regulation in cultured endothelial cells, in a preclinical model and in human tissues was performed and correlated to leukocyte infiltration.
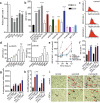
Results: It is shown that treatment of RCC patients with the drugs sunitinib or bevacizumab overcomes tumor endothelial cell anergy. This treatment resulted in an augmented inflammatory state of the tumor, characterized by enhanced infiltration of all major leukocyte subsets, including T cells, regulatory T cells, macrophages of both M1- and M2-like phenotypes and activated dendritic cells. In vitro, exposure of angiogenic endothelial cells to anti-angiogenic drugs normalized ICAM-1 expression. In addition, a panel of tyrosine kinase inhibitors was shown to increase transendothelial migration of both non-adherent and monocytic leukocytes. In primary tumors of RCC patients, ICAM-1 expression was found to be significantly increased in both the sunitinib and bevacizumab-treated groups. Genomic analysis confirmed the correlation between increased immune cell infiltration and ICAM-1 expression upon VEGF-targeted treatment.
Conclusion: The results support the emerging concept that anti-angiogenic therapy can boost immunity and show how immunotherapy approaches can benefit from combination with anti-angiogenic compounds.
Keywords: Angiogenesis; ICAM-1; Leukocyte infiltration; Sunitinib; Tumor endothelial cell anergy.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests.
Figures



References
-
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52(2):237–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

