Methane Oxidation to Methanol
- PMID: 36459432
- PMCID: PMC10176486
- DOI: 10.1021/acs.chemrev.2c00439
Methane Oxidation to Methanol
Abstract
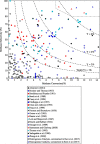
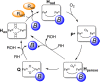
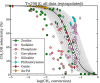
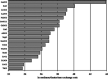
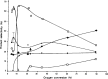
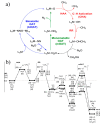
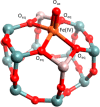
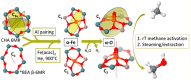
The direct transformation of methane to methanol remains a significant challenge for operation at a larger scale. Central to this challenge is the low reactivity of methane at conditions that can facilitate product recovery. This review discusses the issue through examination of several promising routes to methanol and an evaluation of performance targets that are required to develop the process at scale. We explore the methods currently used, the emergence of active heterogeneous catalysts and their design and reaction mechanisms and provide a critical perspective on future operation. Initial experiments are discussed where identification of gas phase radical chemistry limited further development by this approach. Subsequently, a new class of catalytic materials based on natural systems such as iron or copper containing zeolites were explored at milder conditions. The key issues of these technologies are low methane conversion and often significant overoxidation of products. Despite this, interest remains high in this reaction and the wider appeal of an effective route to key products from C-H activation, particularly with the need to transition to net carbon zero with new routes from renewable methane sources is exciting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


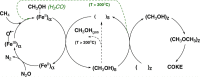
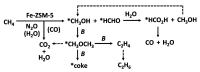
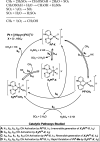
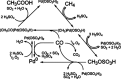
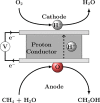
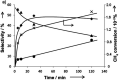


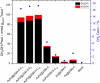
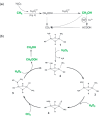
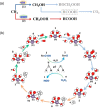
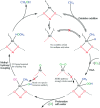

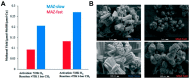
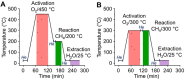



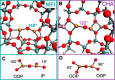

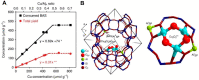
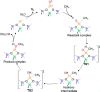

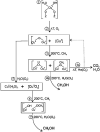

References
-
- Energy Outlook; BP, 2022; https://www.bp.com/en/global/corporate/energy-economics/energy-outlook.html (accessed 2022-04-09).
-
- Sheldon D. Methanol Production - A Technical History. Johnson Matthey Technology Review 2017, 61, 172–182. 10.1595/205651317X695622. - DOI
-
- LNG Market Trends and Their Implications; IEA, 2019; https://www.iea.org/reports/lng-market-trends-and-their-implications (accessed 2021-08-29).
-
- World Energy Outlook 2021; IEA; https://www.iea.org/reports/world-energy-outlook-2021 (accessed 2022-02-08).
-
- Understanding Global Warming Potentials; EPA, 2022; https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed 2022-04-07).
Publication types
LinkOut - more resources
Full Text Sources

