Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells
- PMID: 36459542
- PMCID: PMC9832918
- DOI: 10.1126/sciimmunol.add3075
Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells
Abstract
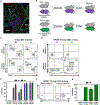
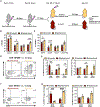
Respiratory tract resident memory T cells (TRM), typically generated by local vaccination or infection, can accelerate control of pulmonary infections that evade neutralizing antibody. It is unknown whether mRNA vaccination establishes respiratory TRM. We generated a self-amplifying mRNA vaccine encoding the influenza A virus nucleoprotein that is encapsulated in modified dendron-based nanoparticles. Here, we report how routes of immunization in mice, including contralateral versus ipsilateral intramuscular boosts, or intravenous and intranasal routes, influenced influenza-specific cell-mediated and humoral immunity. Parabiotic surgeries revealed that intramuscular immunization was sufficient to establish CD8 TRM in the lung and draining lymph nodes. Contralateral, compared with ipsilateral, intramuscular boosting broadened the distribution of lymph node TRM and T follicular helper cells but slightly diminished resulting levels of serum antibody. Intranasal mRNA delivery established modest circulating CD8 and CD4 T cell memory but augmented distribution to the respiratory mucosa. Combining intramuscular immunizations with an intranasal mRNA boost achieved high levels of both circulating T cell memory and lung TRM. Thus, routes of mRNA vaccination influence humoral and cell-mediated immunity, and intramuscular prime-boosting establishes lung TRM that can be further expanded by an additional intranasal immunization.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

