MacroH2A impedes metastatic growth by enforcing a discrete dormancy program in disseminated cancer cells
- PMID: 36459552
- PMCID: PMC10936054
- DOI: 10.1126/sciadv.abo0876
MacroH2A impedes metastatic growth by enforcing a discrete dormancy program in disseminated cancer cells
Abstract
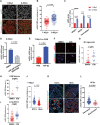
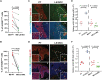
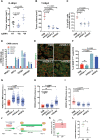
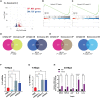
MacroH2A variants have been linked to inhibition of metastasis through incompletely understood mechanisms. Here, we reveal that solitary dormant disseminated cancer cells (DCCs) display increased levels of macroH2A variants in head and neck squamous cell carcinoma PDX in vivo models and patient samples compared to proliferating primary or metastatic lesions. We demonstrate that dormancy-inducing transforming growth factor-β2 and p38α/β pathways up-regulate macroH2A expression and that macroH2A variant overexpression is sufficient to induce DCC dormancy and suppress metastasis in vivo. Notably, inducible expression of the macroH2A2 variant in vivo suppresses metastasis via a reversible growth arrest of DCCs. This state does not require the dormancy-regulating transcription factors DEC2 and NR2F1; instead, transcriptomic analysis reveals that macroH2A2 overexpression inhibits cell cycle and oncogenic signaling programs, while up-regulating dormancy and senescence-associated inflammatory cytokines. We conclude that the macroH2A2-enforced dormant phenotype results from tapping into transcriptional programs of both quiescence and senescence to limit metastatic outgrowth.
Figures
References
-
- Phan T. G., Croucher P. I., The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020). - PubMed
-
- Crist S. B., Ghajar C. M., When a house is not a home: A survey of antimetastatic niches and potential mechanisms of disseminated tumor cell suppression. Annu. Rev. Pathol. 16, 409–432 (2021). - PubMed
-
- M. S. Sosa, E. Bernstein, J. A. Aguirre-Ghiso, Epigenetic regulation of cancer dormancy as a plasticity mechanism for metastasis initiation, in Tumor Dormancy and Recurrence. Y. Wang, F. Crea, Eds. (Springer International Publishing, 2017), pp. 1–16.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

