Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala
- PMID: 36459641
- PMCID: PMC9894161
- DOI: 10.1073/pnas.2213120119
Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala
Abstract
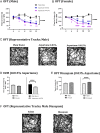
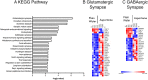
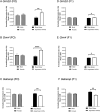
We report the effects of aspartame on anxiety-like behavior, neurotransmitter signaling and gene expression in the amygdala, a brain region associated with the regulation of anxiety and fear responses. C57BL/6 mice consumed drinking water containing 0.015% or 0.03% aspartame, a dose equivalent of 8 to 15% of the FDA recommended maximum human daily intake, or plain drinking water. Robust anxiety-like behavior (evaluated using open field test and elevated zero maze) was observed in male and female mice consuming the aspartame-containing water. Diazepam, an allosteric modulator of the GABA-A receptor, alleviated the anxiety-like behavior. RNA sequencing of the amygdala followed by KEGG biological pathway analysis of differentially expressed genes showed glutamatergic and GABAergic synapse pathways as significantly enriched. Quantitative PCR showed upregulation of mRNA for the glutamate NMDA receptor subunit 2D (Grin2d) and metabotropic receptor 4 (Grm4) and downregulation of the GABA-A receptor associated protein (Gabarap) mRNA. Thus, taken together, our diazepam and gene expression data show that aspartame consumption shifted the excitation-inhibition equilibrium in the amygdala toward excitation. Even more strikingly, the anxiety-like behavior, its response to diazepam, and changes in amygdala gene expression were transmitted to male and female offspring in two generations descending from the aspartame-exposed males. Extrapolation of the findings to humans suggests that aspartame consumption at doses below the FDA recommended maximum daily intake may produce neurobehavioral changes in aspartame-consuming individuals and their descendants. Thus, human population at risk of aspartame's potential mental health effects may be larger than current expectations, which only include aspartame-consuming individuals.
Keywords: artificial sweetener; emotional behavior; intergenerational transmission.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Concern over differences between experimental design and data analyses in Jones et al.Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2302944120. doi: 10.1073/pnas.2302944120. Epub 2023 Jun 5. Proc Natl Acad Sci U S A. 2023. PMID: 37276402 Free PMC article. No abstract available.
References
-
- FDA, Aspartame, commissioner’s final decision. ed D. o. H. a. H. Services (Federal Register, Washington, D.C., 1981), 46 pp. 3824–38308.
-
- Singh R., Singh A., Sachan S. “Enzymes Used in the Food Industry: Friends or Foes?” in Enzymes in Food Biotechnology: Production, Applications, and Future Prospects, Kuddus M. Ed. (Academic Press, London, U.K., 2019), chap. 48, pp. 827–843, 10.1016/B978-0-12-813280-7.09993-X. - DOI
-
- Yokogoshi H., Roberts C. H., Caballero B., Wurtman R. J., Effects of aspartame and glucose administration on brain and plasma levels of large neutral amino acids and brain 5-hydroxyindoles. Am. J. Clin. Nutr. 40, 1–7 (1984). - PubMed
-
- Torii K., Mimura T., Takasaki Y., Ichimura M., Dietary aspartame with protein on plasma and brain amino acids, brain monoamines and behavior in rats. Physiol. Behav. 36, 765–771 (1986). - PubMed
-
- Yokogoshi H., Wurtman R. J., Acute effects of oral or parenteral aspartame on catecholamine metabolism in various regions of rat brain. J. nutrition. 116, 356–364 (1986). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

