Specific inhibition of NADPH oxidase 2 modifies chronic epilepsy
- PMID: 36459714
- PMCID: PMC9712695
- DOI: 10.1016/j.redox.2022.102549
Specific inhibition of NADPH oxidase 2 modifies chronic epilepsy
Abstract
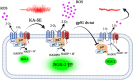
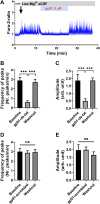
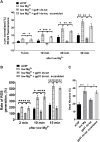
Recent work by us and others has implicated NADPH oxidase (NOX) enzymes as main producers of reactive oxygen species (ROS) following a brain insult such as status epilepticus, contributing to neuronal damage and development of epilepsy. Although several NOX isoforms have been examined in the context of epilepsy, most attention has focused on NOX2. In this present study, we demonstrate the effect of gp91ds-tat, a specific competitive inhibitor of NOX2, in in vitro epileptiform activity model as well as in temporal lobe epilepsy (TLE) model in rats. We showed that in in vitro seizure model, gp91ds-tat modulated Ca2+ oscillation, prevented epileptiform activity-induced ROS generation, mitochondrial depolarization, and neuronal death. Administration of gp91ds-tat 1 h after kainic acid-induced status epilepticus significantly decreased the expression of NOX2, as well as the overall NOX activity in the cortex and the hippocampus. Finally, we showed that upon continuous intracerebroventricular administration to epileptic rats, gp91ds-tat significantly reduced the seizure frequency and the total number of seizures post-treatment compared to the scrambled peptide-treated animals. The results of the study suggest that NOX2 may have an important effect on modulation of epileptiform activity and has a critical role in mediating seizure-induced NOX activation, ROS generation and oxidative stress in the brain, and thus significantly contributes to development of epilepsy following a brain insult.
Keywords: NOX2; Reactive oxygen species; Status epilepticus; Temporal lobe epilepsy; gp91ds-tat.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest All authors declared that they have no competing interest in connection with this manuscript.
Figures


References
-
- Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. - PubMed
-
- Zhang R., et al. Nrf2—a promising therapeutic target for defensing against oxidative stress in stroke. Mol. Neurobiol. 2017;54(8):6006–6017. - PubMed
-
- Bhatti J., et al. Systematic review of human and animal studies examining the efficacy and safety of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) in traumatic brain injury: impact on neurofunctional outcome and biomarkers of oxidative stress and inflammation. Front. Neurol. 2017;8:744. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

