Free HIV self-test for identification and linkage to care of previously undetected HIV infection in men who have sex with men in England and Wales (SELPHI): an open-label, internet-based, randomised controlled trial
- PMID: 36460023
- PMCID: PMC7614584
- DOI: 10.1016/S2352-3018(22)00266-1
Free HIV self-test for identification and linkage to care of previously undetected HIV infection in men who have sex with men in England and Wales (SELPHI): an open-label, internet-based, randomised controlled trial
Abstract
Background: High levels of HIV testing in men who have sex with men remain key to reducing the incidence of HIV. We aimed to assess whether the offer of a single, free HIV self-testing kit led to increased HIV diagnoses with linkage to care.
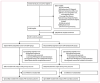
Methods: SELPHI was an internet-based, open-label, randomised controlled trial that recruited participants via sexual and social networking sites. Eligibility criteria included being a man or trans woman (although trans women are reported separately); being resident in England or Wales, UK; being aged 16 years or older; having had anal intercourse with a man; not having a positive HIV diagnosis; and being willing to provide name, email address, date of birth, and consent to link to national HIV databases. Participants were randomly allocated (3:2) by computer-generated number sequence to receive a free HIV self-test kit (BT group) or to not receive this free kit (nBT group). Online surveys collected data at baseline, 2 weeks after enrolment (BT group only), 3 months after enrolment, and at the end of the study. The primary outcome was confirmed (linked to care) new HIV diagnosis within 3 months of enrolment, analysed by intention to treat. Those assessing the primary outcome were masked to allocation. This study is registered with the ISRCTN Clinical Trials Register, number ISRCTN20312003.
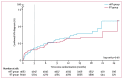
Findings: 10 111 participants (6049 in BT group and 4062 in nBT group) enrolled between Feb 16, 2017, and March 1, 2018. The median age of participants was 33 years (IQR 26-44 years); 9000 (89%) participants were White; 8118 (80%) participants were born in the UK; 81 (1%) participants were transgender men; 4706 (47%) participants were university educated; 1537 (15%) participants had never been tested for HIV; and 389 (4%) participants were taking pre-exposure prophylaxis. At enrolment, 7282 (72%) participants reported condomless anal sex with at least one male partner in the previous 3 months. In the BT group, of the 4511 participants for whom HIV testing information was available, 4263 (95%) reported having used the free HIV self-test kit within 3 months.Within 3 months of enrolment there were 19 confirmed new HIV diagnoses (0·31%) in 6049 participants in the BT group and 15 (0·37%) of 4062 in the nBT group (p=0·64).
Interpretation: The offer of a single, free HIV self-test did not lead to increased rates of new HIV diagnoses, which could reflect decreasing HIV incidence rates in the UK. Nonetheless, the offer of a free HIV self-testing kit resulted in high HIV testing rates, indicating that self-testing is an attractive testing option for a large group of men who have sex with men.
Funding: UK National Institute for Health and Care Research.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests MB has received speaker's fees from Gilead and Bristol Myers Squibb. FB and TCW have received consultancy fees from Gilead. All other authors declare no competing interests.
Figures
Comment in
-
HIV self-testing: from availability to strategic deployment.Lancet HIV. 2022 Dec;9(12):e811-e812. doi: 10.1016/S2352-3018(22)00333-2. Lancet HIV. 2022. PMID: 36460020 No abstract available.
References
-
- Girometri N, Delpech V, McCormack S, et al. The success of HIV combination prevention: the Dean street model. HIV Med. 2021;22:892–97. - PubMed
-
- Public Health England. Trends in HIV testing, new diagnoses and people receiving HIV-related care in the United Kingdom: data to the end of December 2019. 2020. [accessed May 10, 2022]. https://assets.publishing.service.gov.uk/government/uploads/system/uploa... .
-
- O’Halloran C, Sun S, Nash S, et al. HIV in the United Kingdom: towards zero 2030. 2019. [accessed May 10, 2022]. https://assets.publishing.service.gov.uk/government/uploads/system/uploa... .
-
- Brown AE, Nash S, Connor N, et al. Towards elimination of HIV transmission, AIDS and HIV-related deaths in the UK. HIV Med. 2018;20:74–76. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

