Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: a multicentre, open-label, single-arm, phase 2/3 trial
- PMID: 36460027
- PMCID: PMC10033454
- DOI: 10.1016/S1473-3099(22)00660-0
Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: a multicentre, open-label, single-arm, phase 2/3 trial
Erratum in
-
Correction to Lancet Infect Dis 2022; published online Nov 29. https://doi.org/10.1016/S1473-3099(22)00660-0.Lancet Infect Dis. 2023 Feb;23(2):e47. doi: 10.1016/S1473-3099(22)00859-3. Epub 2022 Dec 19. Lancet Infect Dis. 2023. PMID: 36549309 Free PMC article. No abstract available.
Abstract
Background: Human African trypanosomiasis caused by Trypanosoma brucei gambiense (gambiense HAT) in patients with late-stage disease requires hospital admission to receive nifurtimox-eflornithine combination therapy (NECT). Fexinidazole, the latest treatment that has been recommended by WHO, also requires systematic admission to hospital, which is problematic in areas with few health-care resources. We aim to assess the safety and efficacy of acoziborole in adult and adolescent patients with gambiense HAT.
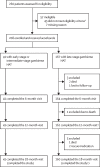
Methods: This multicentre, prospective, open-label, single-arm, phase 2/3 study recruited patients aged 15 years or older with confirmed gambiense HAT infection from ten hospitals in the Democratic Republic of the Congo and Guinea. Inclusion criteria included a Karnofsky score greater than 50, ability to swallow tablets, a permanent address or traceability, ability to comply with follow-up visits and study requirements, and agreement to hospital admission during treatment. Oral acoziborole was administered as a single 960 mg dose (3 × 320 mg tablets) to fasted patients. Patients were observed in hospital until day 15 after treatment administration then for 18 months as outpatients with visits at 3, 6, 12, and 18 months. The primary efficacy endpoint was the success rate of acoziborole treatment at 18 months in patients with late-stage gambiense HAT (modified intention-to-treat [mITT] population), based on modified WHO criteria. A complementary post-hoc analysis comparing the 18-month success rates for acoziborole and NECT (using historical data) was performed. This study is registered at ClinicalTrials.gov, NCT03087955.
Findings: Between Oct 11, 2016, and March 25, 2019, 260 patients were screened, of whom 52 were ineligible and 208 were enrolled (167 with late-stage and 41 with early-stage or intermediate-stage gambiense HAT; primary efficacy analysis set). All 41 (100%) patients with early-stage or intermediate-stage and 160 (96%) of 167 with late-stage disease completed the last 18-month follow-up visit. The mean age of participants was 34·0 years (SD 12·4), including 117 (56%) men and 91 (44%) women. Treatment success rate at 18 months was 95·2% (95% CI 91·2-97·7) reached in 159 of 167 patients with late-stage gambiense HAT (mITT population) and 98·1% (95·1-99·5) reached in 159 of 162 patients (evaluable population). Overall, 155 (75%) of 208 patients had 600 treatment-emergent adverse events. A total of 38 drug-related treatment-emergent adverse events occurred in 29 (14%) patients; all were mild or moderate and most common were pyrexia and asthenia. Four deaths occurred during the study; none were considered treatment related. The post-hoc analysis showed similar results to the estimated historical success rate for NECT of 94%.
Interpretation: Given the high efficacy and favourable safety profile, acoziborole holds promise in the efforts to reach the WHO goal of interrupting HAT transmission by 2030.
Funding: Bill & Melinda Gates Foundation, UK Aid, Federal Ministry of Education and Research, Swiss Agency for Development and Cooperation, Médecins Sans Frontières, Dutch Ministry of Foreign Affairs, Norwegian Agency for Development Cooperation, Norwegian Ministry of Foreign Affairs, the Stavros Niarchos Foundation, Spanish Agency for International Development Cooperation, and the Banco Bilbao Vizcaya Argentaria Foundation.
Translation: For the French translation of the abstract see Supplementary Materials section.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests BS reports fees from the Drugs for Neglected Diseases initiative (DNDi) for the statistical report and consulting fees from CEMAG, D&A Pharma, Inventiva, and OT4B Pharma. WMK, SR, AP, OVM, NS-W, DNT, and AT report employment at the DNDi. SS declares that Swiss Tropical and Public Health Institute acted as a service provider for the DNDi by monitoring the study sites. All other authors declare no competing interests.
Figures
Comment in
-
Sleeping sickness: time for dreaming.Lancet Infect Dis. 2023 Apr;23(4):387-388. doi: 10.1016/S1473-3099(22)00686-7. Epub 2022 Nov 29. Lancet Infect Dis. 2023. PMID: 36460028 No abstract available.
References
-
- Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. - PubMed
-
- WHO Control and surveillance of human African trypanosomiasis: report of a WHO expert committee. 2013. https://apps.who.int/iris/handle/10665/95732
-
- Bottieau E, Clerinx J. Human African trypanosomiasis: progress and stagnation. Infect Dis Clin North Am. 2019;33:61–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

