Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters
- PMID: 36460750
- PMCID: PMC9734468
- DOI: 10.1007/s00018-022-04643-1
Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters
Abstract
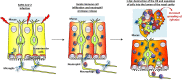
The loss of smell (anosmia) related to SARS-CoV-2 infection is one of the most common symptoms of COVID-19. Olfaction starts in the olfactory epithelium mainly composed of olfactory sensory neurons surrounded by supporting cells called sustentacular cells. It is now clear that the loss of smell is related to the massive infection by SARS-CoV-2 of the sustentacular cells in the olfactory epithelium leading to its desquamation. However, the molecular mechanism behind the destabilization of the olfactory epithelium is less clear. Using golden Syrian hamsters infected with an early circulating SARS-CoV-2 strain harboring the D614G mutation in the spike protein; we show here that rather than being related to a first wave of apoptosis as proposed in previous studies, the innate immune cells play a major role in the destruction of the olfactory epithelium. We observed that while apoptosis remains at a low level in the damaged area of the infected epithelium, the latter is invaded by Iba1+ cells, neutrophils and macrophages. By depleting the neutrophil population or blocking the activity of neutrophil elastase-like proteinases, we could reduce the damage induced by the SARS-CoV-2 infection. Surprisingly, the impairment of neutrophil activity led to a decrease in SARS-CoV-2 infection levels in the olfactory epithelium. Our results indicate a counterproductive role of neutrophils leading to the release of infected cells in the lumen of the nasal cavity and thereby enhanced spreading of the virus in the early phase of the SARS-CoV-2 infection.
Keywords: Innate immunity; Pathophysiology; Post-viral olfactory disorder (PVOD).
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
All authors do not have conflict of interest.
Figures


Similar articles
-
Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters.Brain Behav Immun. 2020 Oct;89:579-586. doi: 10.1016/j.bbi.2020.06.032. Epub 2020 Jul 3. Brain Behav Immun. 2020. PMID: 32629042 Free PMC article.
-
Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters.ACS Chem Neurosci. 2021 Feb 17;12(4):589-595. doi: 10.1021/acschemneuro.0c00649. Epub 2021 Feb 1. ACS Chem Neurosci. 2021. PMID: 33522795 Free PMC article.
-
COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters.Sci Transl Med. 2021 Jun 2;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396. Epub 2021 May 3. Sci Transl Med. 2021. PMID: 33941622 Free PMC article.
-
The Molecular Basis of Olfactory Dysfunction in COVID-19 and Long COVID.Lifestyle Genom. 2024;17(1):42-56. doi: 10.1159/000539292. Epub 2024 May 15. Lifestyle Genom. 2024. PMID: 38749402 Review.
-
Anosmia: an evolution of our understanding of its importance in COVID-19 and what questions remain to be answered.Eur Arch Otorhinolaryngol. 2021 Jul;278(7):2187-2191. doi: 10.1007/s00405-020-06285-0. Epub 2020 Sep 9. Eur Arch Otorhinolaryngol. 2021. PMID: 32909060 Free PMC article. Review.
Cited by
-
Mechanisms of SARS-CoV-2-associated anosmia.Physiol Rev. 2023 Oct 1;103(4):2759-2766. doi: 10.1152/physrev.00012.2023. Epub 2023 Jun 21. Physiol Rev. 2023. PMID: 37342077 Free PMC article. Review.
-
Inflammatory Response and Defects on Myelin Integrity in the Olfactory System of K18hACE2 Mice Infected with SARS-CoV-2.eNeuro. 2024 Jun 17;11(6):ENEURO.0106-24.2024. doi: 10.1523/ENEURO.0106-24.2024. Print 2024 Jun. eNeuro. 2024. PMID: 38834299 Free PMC article.
-
Olfactory and trigeminal routes of HSV-1 CNS infection with regional microglial heterogeneity.J Virol. 2024 Nov 19;98(11):e0096824. doi: 10.1128/jvi.00968-24. Epub 2024 Oct 30. J Virol. 2024. PMID: 39475273 Free PMC article.
-
Histochemical Evidence for Reduced Immune Response in Nasal Mucosa of Patients with COVID-19.Int J Mol Sci. 2024 Apr 17;25(8):4427. doi: 10.3390/ijms25084427. Int J Mol Sci. 2024. PMID: 38674011 Free PMC article.
-
Olfactory immune response to SARS-CoV-2.Cell Mol Immunol. 2024 Feb;21(2):134-143. doi: 10.1038/s41423-023-01119-5. Epub 2023 Dec 25. Cell Mol Immunol. 2024. PMID: 38143247 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

