Cardiac xenotransplantation: from concept to clinic
- PMID: 36461918
- PMCID: PMC9897693
- DOI: 10.1093/cvr/cvac180
Cardiac xenotransplantation: from concept to clinic
Abstract
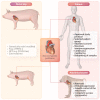
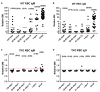
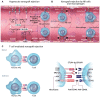
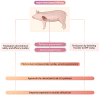
For many patients with terminal/advanced cardiac failure, heart transplantation is the most effective, durable treatment option, and offers the best prospects for a high quality of life. The number of potentially life-saving donated human organs is far fewer than the population who could benefit from a new heart, resulting in increasing numbers of patients awaiting replacement of their failing heart, high waitlist mortality, and frequent reliance on interim mechanical support for many of those deemed among the best candidates but who are deteriorating as they wait. Currently, mechanical assist devices supporting left ventricular or biventricular heart function are the only alternative to heart transplant that is in clinical use. Unfortunately, the complication rate with mechanical assistance remains high despite advances in device design and patient selection and management, and the quality of life of the patients even with good outcomes is only moderately improved. Cardiac xenotransplantation from genetically multi-modified (GM) organ-source pigs is an emerging new option as demonstrated by the consistent long-term success of heterotopic (non-life-supporting) abdominal and life-supporting orthotopic porcine heart transplantation in baboons, and by a recent 'compassionate use' transplant of the heart from a GM pig with 10 modifications into a terminally ill patient who survived for 2 months. In this review, we discuss pig heart xenotransplantation as a concept, including pathobiological aspects related to immune rejection, coagulation dysregulation, and detrimental overgrowth of the heart, as well as GM strategies in pigs to prevent or minimize these problems. Additional topics discussed include relevant results of heterotopic and orthotopic heart transplantation experiments in the pig-to-baboon model, microbiological and virologic safety concepts, and efficacy requirements for initiating formal clinical trials. An adequate regulatory and ethical framework as well as stringent criteria for the selection of patients will be critical for the safe clinical development of cardiac xenotransplantation, which we expect will be clinically tested during the next few years.
Keywords: Heart; Non-human primate; Pig; Xenotransplantation clinical; Xenotransplantation experimental.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: B.R., M.L., and E.W. are cofounders of XTransplant GmbH, Starnberg, Germany. D.K.C.C. is a paid consultant to eGenesis. D.K.C.C. and R.N.P. have previously received research support from Revivicor, Lung Bioengineering PBC, and United Therapeutics; R.N.P. receives research support from eGenesis and Tonix.
Figures



References
-
- Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: A review. JAMA 2020;324:488–504. - PubMed
-
- Khush KK, Potena L, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, Sadavarte A, Singh TP, Zuckermann A, Stehlik J. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1003–1015. - PMC - PubMed
-
- Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 years of clinical heart transplantation in circulation: in-depth state-of-the-art review. Circulation 2018;137:71–87. - PubMed
-
- Kittleson MM, Kobashigawa JA. Cardiac transplantation: current outcomes and contemporary controversies. JACC Heart Fail 2017;5:857–868. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

