Antepartum SARS-CoV-2 infection and adverse birth outcomes in South African women
- PMID: 36462199
- PMCID: PMC9718447
- DOI: 10.7189/jogh.12.05050
Antepartum SARS-CoV-2 infection and adverse birth outcomes in South African women
Abstract
Background: SARS-CoV-2 infection in pregnant women has been associated with severe illness in the women and higher rates of premature delivery. There is, however, paucity of data on the impact of the timing of SARS-CoV-2 infection and on symptomatic or asymptomatic infections on birth outcomes. Data from low-middle income settings is also lacking.
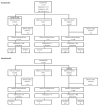
Methods: We conducted a longitudinal study from April 2020 to March 2021, in South Africa, where symptomatic or asymptomatic pregnant women were investigated for SARS-CoV-2 infection during the antepartum period. We aimed to evaluate if there was an association between antepartum SARS-CoV-2 infection on birth outcomes. SARS-CoV-2 infection was investigated by nucleic acid amplification test (NAAT), histological examination was performed in a sub-set of placentas.
Results: Overall, 793 women were tested for SARS-CoV-2 antenatally, including 275 (35%) who were symptomatic. SARS-CoV-2 infection was identified in 138 (17%) women, of whom 119 had symptoms (COVID-19 group) and 19 were asymptomatic. The 493 women who were asymptomatic and had a negative SARS-CoV-2 NAAT were used as the referent comparator group for outcomes evaluation. Women with COVID-19 compared with the referent group were 1.66-times (95% confidence interval (CI) = 1.02-2.71) more likely to have a low-birthweight newborn (30% vs 21%) and 3.25-times more likely to deliver a very low-birthweight newborn (5% vs 2%). Similar results for low-birthweight were obtained comparing women with SARS-CoV-2 confirmed infection (30%) with those who had a negative NAAT result (22%) independent of symptoms presentation. The placentas from women with antenatal SARS-CoV-2 infection had higher percentage of chorangiosis (odds ratio (OR) = 3.40, 95% CI = 1.18-.84), while maternal vascular malperfusion was more frequently identified in women who tested negative for SARS-CoV-2 (aOR = 0.28, 95% CI = 0.09-0.89).
Conclusions: Our study demonstrates that in a setting with high HIV infection prevalence and other comorbidities antenatal SARS-CoV-2 infection was associated with low-birthweight delivery.
Copyright © 2022 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form. MCN reports grants to her institution from the Bill & Melinda Gates Foundation, European & Developing Countries Clinical Trials Partnership, Pfizer, AstraZeneca and Sanofi. Personal honoraria received from Pfizer and Sanofi unrelated to the manuscript. SAM reports grants to his institution related to COVID-19 epidemiology and vaccine studies from the Bill & Melinda Gates Foundation, South African Medical Research Council, Novavax, Pfizer, Gritstone (PATH), Providence, Johnson and Johnson, AstraZeneca and European & Developing Countries Clinical Trials Partnership. Additional non-COVID-19 grants to the institution also received from GSK and Minervax. Personal honoraria received from Bill & Melinda Gates Foundation unrelated to the manuscript. Other authors disclose no relevant interests.
Figures
References
-
- WHO. COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available: https://covid19.who.int/. Accessed: 30 June 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous