Stable Cuprous Hydroxide Nanostructures by Organic Ligand Functionalization
- PMID: 36462218
- PMCID: PMC9975062
- DOI: 10.1002/adma.202208665
Stable Cuprous Hydroxide Nanostructures by Organic Ligand Functionalization
Abstract
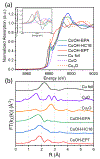
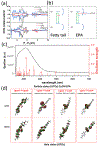
Copper compounds have been extensively investigated for diverse applications. However, studies of cuprous hydroxide (CuOH) have been scarce due to structural metastability. Herein, a facile, wet-chemistry procedure is reported for the preparation of stable CuOH nanostructures via deliberate functionalization with select organic ligands, such as acetylene and mercapto derivatives. The resulting nanostructures are found to exhibit a nanoribbon morphology consisting of small nanocrystals embedded within a largely amorphous nanosheet-like scaffold. The acetylene derivatives are found to anchor onto the CuOH forming CuC linkages, whereas CuS interfacial bonds are formed with the mercapto ligands. Effective electronic coupling occurs at the ligand-core interface in the former, in contrast to mostly non-conjugated interfacial bonds in the latter, as manifested in spectroscopic measurements and confirmed in theoretical studies based on first principles calculations. Notably, the acetylene-capped CuOH nanostructures exhibit markedly enhanced photodynamic activity in the inhibition of bacteria growth, as compared to the mercapto-capped counterparts due to a reduced material bandgap and effective photocatalytic generation of reactive oxygen species. Results from this study demonstrate that deliberate structural engineering with select organic ligands is an effective strategy in the stabilization and functionalization of CuOH nanostructures, a critical first step in exploring their diverse applications.
Keywords: acetylene; antimicrobial activity; cuprous hydroxide; interfacial electronic coupling; mercapto ligands.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures





References
-
- Yin M, Wu CK, Lou YB, Burda C, Koberstein JT, Zhu YM, O’Brien S, J. Am. Chem. Soc 2005, 127, 9506. - PubMed
-
- Golden TD, Shumsky MG, Zhou YC, VanderWerf RA, VanLeeuwen RA, Switzer JA, Chem. Mater. 1996, 8, 2499.
-
- Wu XF, Bai H, Zhang JX, Chen FE, Shi GQ, J. Phys. Chem. B 2005, 109, 22836. - PubMed
-
- Peng XS, Jin J, Ichinose I, Adv. Funct. Mater. 2007, 17, 1849.
-
- Saldanha PL, Brescia R, Prato M, Li HB, Povia M, Manna L, Lesnyak V, Chem. Mater. 2014, 26, 1442.

