Quantitative artifact reduction and pharmacologic paralysis improve detection of EEG epileptiform activity in critically ill patients
- PMID: 36462473
- PMCID: PMC9897212
- DOI: 10.1016/j.clinph.2022.11.007
Quantitative artifact reduction and pharmacologic paralysis improve detection of EEG epileptiform activity in critically ill patients
Abstract
Objective: Epileptiform activity is common in critically ill patients, but movement-related artifacts-including electromyography (EMG) and myoclonus-can obscure EEG, limiting detection of epileptiform activity. We sought to determine the ability of pharmacologic paralysis and quantitative artifact reduction (AR) to improve epileptiform discharge detection.
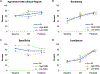
Methods: Retrospective analysis of patients who underwent continuous EEG monitoring with pharmacologic paralysis. Four reviewers read each patient's EEG pre- and post- both paralysis and AR, and indicated the presence of epileptiform discharges. We compared the interrater reliability (IRR) of identifying discharges at baseline, post-AR, and post-paralysis, and compared the performance of AR and paralysis according to artifact type.
Results: IRR of identifying epileptiform discharges at baseline was slight (N = 30; κ = 0.10) with a trend toward increase post-AR (κ = 0.26, p = 0.053) and a significant increase post-paralysis (κ = 0.51, p = 0.001). AR was as effective as paralysis at improving IRR of identifying discharges in those with high EMG artifact (N = 15; post-AR κ = 0.63, p = 0.009; post-paralysis κ = 0.62, p = 0.006) but not with primarily myoclonus artifact (N = 15).
Conclusions: Paralysis improves detection of epileptiform activity in critically ill patients when movement-related artifact obscures EEG features. AR improves detection as much as paralysis when EMG artifact is high, but is ineffective when the primary source of artifact is myoclonus.
Significance: In the appropriate setting, both AR and paralysis facilitate identification of epileptiform activity in critically ill patients.
Keywords: Continuous video EEG; EEG interpretation; Myoclonus; Neurocritical care.
Copyright © 2022 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Figures



References
-
- Artifact Reduction. Persyst Dev Corp. https://www.persyst.com/technology/artifact-reduction/; 2022.
-
- Clar DT, Liu M. Non-depolarizing neuromuscular blockers. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
-
- Debaene B, Plaud B, Dilly M-P, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. J Am Soc Anesthesiol 2003;98:1042–8. - PubMed
-
- Field CA, Welsh AH. Bootstrapping clustered data. J R Stat Soc Series B Stat Methodol 2007;69:369–90.
-
- Fitzgibbon SP, DeLosAngeles D, Lewis TW, Powers DMW, Grummett TS, Whitham EM, et al. Automatic determination of EMG-contaminated components and validation of independent component analysis using EEG during pharmacologic paralysis. Clin Neurophysiol 2016;127:1781–93. 10.1016/j.clinph.2015.12.009. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

