Vaginal cleansing before unscheduled cesarean delivery to reduce infection: a randomized clinical trial
- PMID: 36462539
- PMCID: PMC10227184
- DOI: 10.1016/j.ajog.2022.11.1300
Vaginal cleansing before unscheduled cesarean delivery to reduce infection: a randomized clinical trial
Abstract
Background: Cesarean delivery is the most performed major surgery among women, and surgical-site infections following a cesarean delivery are a significant source of postoperative morbidity. It is unclear if vaginal cleansing before a cesarean delivery decreases post-cesarean delivery infectious morbidity.
Objective: This study aimed to evaluate if preoperative vaginal cleansing with povidone-iodine among women undergoing a cesarean delivery after labor decreases postoperative infectious morbidity.
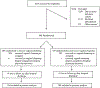
Study design: This randomized clinical trial was conducted from August 3, 2015 to January 28, 2021, with 30 days of follow-up and the final follow-up completed on February 27, 2021. Patients met the inclusion criteria if they underwent a cesarean delivery after regular contractions with cervical dilation, rupture of membranes, or any cesarean delivery performed at >4 cm dilation. Participants were randomly assigned in a 1:1 ratio to either abdominal cleansing plus vaginal cleansing with 1% povidone-iodine or abdominal cleansing alone. The primary outcome was composite infectious morbidity including surgical-site infection, fever, endometritis, and wound complications within 30 days after the cesarean delivery. Secondary outcomes included individual components of the composite, length of hospital stay, postoperative hospitalization or outpatient treatment related to infectious morbidity, and empirical treatment for neonatal sepsis.
Results: A total of 608 subjects (304 vaginal cleansing group, 304 control group) were included in the intention-to-treat analysis. Patient characteristics were similar between groups. There was no significant difference in the primary composite outcome between the 2 groups (11.8% vs 11.5%; P=.90; relative risk, 1.0; 95% confidence interval, 0.7-1.6). Individual components of the composite and secondary outcomes were also not significantly different between the groups. Similar findings were observed in the as-treated analysis (11.3% vs 11.8%; P=.9; relative risk, 1.0; 95% confidence interval, 0.7-1.6).
Conclusion: Vaginal cleansing with povidone-iodine before an unscheduled cesarean delivery occurring after labor did not reduce the postoperative infectious morbidity. These findings do not support the routine use of vaginal cleansing for women undergoing a cesarean delivery after labor.
Trial registration: ClinicalTrials.gov NCT02495753.
Keywords: cesarean delivery; infectious morbidity; operative morbidity; operative obstetrics; postoperative infection; prophylaxis; vaginal cleansing.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Excerpts from the World Obstetrics Literature: Obstetrics.J Obstet Gynaecol Can. 2023 Apr;45(4):249-252. doi: 10.1016/j.jogc.2023.03.002. J Obstet Gynaecol Can. 2023. PMID: 37149337 No abstract available.
-
La littérature médicale mondiale : obstétrique.J Obstet Gynaecol Can. 2023 Apr;45(4):253-256. doi: 10.1016/j.jogc.2023.04.001. J Obstet Gynaecol Can. 2023. PMID: 37149338 No abstract available.
References
-
- Hamilton Brady E., P.D., Martin Joyce A., M.P.H., and Osterman Michelle J.K., M.H.S., Births: Provisional Data for 2020. Vital Statistics Rapid Release, 2021. Report No. 012. - PubMed
-
- Tuuli MG S. MJ : Martin S : Rampersad RM : Cahill AG : Macones GA, Comparison of suture materials for subcuticular skin closure at cesarean delivery. American Journal of Obstetrics and Gynecology, 2016. 215(4): p. 490.e1–490.e5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous