iTat transgenic mice exhibit hyper-locomotion in the behavioral pattern monitor after chronic exposure to methamphetamine but are unaffected by Tat expression
- PMID: 36462584
- PMCID: PMC10014034
- DOI: 10.1016/j.pbb.2022.173499
iTat transgenic mice exhibit hyper-locomotion in the behavioral pattern monitor after chronic exposure to methamphetamine but are unaffected by Tat expression
Abstract
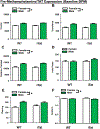
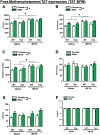
Although antiretroviral therapy (ART) has increased the quality of life and lifespan in people living with HIV (PWH), millions continue to suffer from the neurobehavioral effects of the virus. Additionally, the abuse of illicit drugs (methamphetamine in particular) is significantly higher in PWH compared to the general population, which may further impact their neurological functions. The HIV regulatory protein, Tat, has been implicated in the neurobehavioral impacts of HIV and is purported to inhibit dopamine transporter (DAT) function in a way similar to methamphetamine. Thus, we hypothesized that a combination of Tat expression and methamphetamine would exert synergistic deleterious effects on behavior and DAT expression. We examined the impact of chronic methamphetamine exposure on exploration in transgenic mice expressing human Tat (iTat) vs. their wildtype littermates using the behavioral pattern monitor (BPM). During baseline, mice exhibited sex-dependent differences in BPM behavior, which persisted through methamphetamine exposure, and Tat activation with doxycycline. We observed a main effect of methamphetamine, wherein exposure, irrespective of genotype, increased locomotor activity and decreased specific exploration. After doxycycline treatment, mice continued to exhibit drug-dependent alterations in locomotion, with no effect of Tat, or methamphetamine interactions. DAT levels were higher in wildtype, saline-exposed males compared to all other groups. These data support stimulant-induced changes of locomotor activity and exploration, and suggest that viral Tat and methamphetamine do not synergistically interact to alter these behaviors in mice. These findings are important for future studies attempting to disentangle the effect of substances that impact DAT on HAND-relevant behaviors using such transgenic animals.
Keywords: Behavioral pattern monitor; Dopamine; Exploration; Mouse; Movement; iTAT HIV model.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures



References
-
- Appadoo CN, Sambo D, Alonge T, Harvey B, Khoshbouei H, 2017. The dual effect of HIV-1 Tat and methamphetamine on dopamine transporter function. FASEB J 31 (S1), 610–662. 10.1096/fasebj.31.1_supplement.662.10. - DOI
-
- Baek EJ, Kim H, Basova LA, Rosander A, Kesby JP, Semenova S, Marcondes MCG, 2020. Sex differences and tat expression affect dopaminergic receptor expression and response to antioxidant treatment in methamphetamine-sensitized HIV tat transgenic mice. Neuropharmacology 178, 108245. 10.1016/j.neuropharm.2020.108245. - DOI - PMC - PubMed

