Effect of farnesyltransferase inhibitors on SARS-CoV-2
- PMID: 36462736
- PMCID: PMC9711904
- DOI: 10.1016/j.jgar.2022.11.011
Effect of farnesyltransferase inhibitors on SARS-CoV-2
Abstract
Objectives: The emergence of SARS-CoV-2 in 2019 led to a severe pandemic situation. Treatment options are limited, and the efficacy of vaccines decreases due to mutations in SARS-CoV-2 strains. Therefore, new treatment options are urgently needed, and computational compound screenings are used to predict drugs quickly. One of these screenings revealed farnesyltransferase inhibitors (FTIs) as potential candidates.
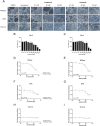
Methods: SARS-CoV-2 infected Calu-3 cells were treated with lonafarnib and tipifarnib and fold change viral replication of SARS-CoV-2 was measured using RT-qPCR. Furthermore, morphological changes, like CPE formation, were evaluated. Effects on Calu-3 cells were analyzed using MTT assay.
Results: We demonstrated that the FTIs lonafarnib and tipifarnib have an effect on SARS-CoV-2 Wildtype and the Delta variant. Both FTIs dose-dependently reduced morphological changes and the formation of cytopathic effects in SARS-CoV-2 infected Calu-3 cells. The effect of the FTIs on Omicron needs to be further elucidated because of inefficient viral replication.
Conclusions: The FTI lonafarnib and tipifarnib might be effective drugs against different SARS-CoV-2 strains.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Competing interests The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167–1174. doi: 10.1016/S1473-3099(15)00074-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

