Non-invasive respiratory support in SARS-CoV-2 related acute respiratory distress syndrome: when is it most appropriate to start treatment?
- PMID: 36463178
- PMCID: PMC9719658
- DOI: 10.1186/s12931-022-02258-5
Non-invasive respiratory support in SARS-CoV-2 related acute respiratory distress syndrome: when is it most appropriate to start treatment?
Abstract
Background: Acute respiratory distress syndrome (ARDS) is one of the most severe complications of SARS-CoV-2 infection. Non-Invasive Respiratory Support (NRS) as Continuous Positive Airway Pressure (CPAP) and/or Non-Invasive Ventilation (NIV) has been proven as effective in the management of SARS-CoV-2-related ARDS. However, the most appropriate timing for start NRS is unknown.
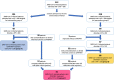
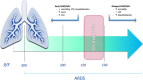
Methods: We conducted a prospective pilot study including all consecutive patients who developed moderate SARS-CoV-2-related ARDS during hospitalization. Patients were randomly divided into two intervention groups according to ARDS severity (assessed by PaO2/FiO2-P/F) at NRS beginning: group A started CPAP/NIV when P/F was ≤ 200 and group B started CPAP/NIV when P/F was ≤ 150. Eligible patients who did not give their consent to CPAP/NIV until the severe stage of ARDS and started non-invasive treatment when P/F ≤ 100 (group C) was added. The considered outcomes were in-hospital mortality, oro-tracheal intubation (OTI) and days of hospitalization.
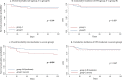
Results: Among 146 eligible patients, 29 underwent CPAP/NIV when P/F was ≤ 200 (Group A), 68 when P/F was ≤ 150 (Group B) and 31 patients agreed to non-invasive treatment only when P/F was ≤ 100 (Group C). Starting NRS at P/F level between 151 and 200 did not results in significant differences in the outcomes as compared to treatment starting with P/F ranging 101-150. Conversely, patients undergone CPAP/NIV in a moderate stage (P/F 101-200) had a significantly lower in-hospital mortality rate (13.4 vs. 29.0%, p = 0.044) and hospitalization length (14 vs. 15 days, p = 0.038) than those in the severe stage (P/F ≤ 100). Age and need for continuous ventilation were independent predictors of CPAP/NIV failure.
Conclusions: Starting CPAP/NIV in patients with SARS-CoV-2-related ARDS in moderate stage (100 > P/F ≤ 200) is associated to a reduction of both in-hospital mortality and hospitalization length compared to the severe stage (P/F ≤ 100). Starting CPAP/NIV with a P/F > 150 does not appear to be of clinical utility.
Keywords: ARDS; COVID-19; CPAP; NIV; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Mortality and clinical outcomes in patients with COVID-19 pneumonia treated with non-invasive respiratory support: A rapid review.J Crit Care. 2021 Oct;65:1-8. doi: 10.1016/j.jcrc.2021.05.007. Epub 2021 May 21. J Crit Care. 2021. PMID: 34052780 Free PMC article. Review.
-
[Position Paper for the State of the Art Application of Respiratory Support in Patients with COVID-19 - German Respiratory Society].Pneumologie. 2020 Jun;74(6):337-357. doi: 10.1055/a-1157-9976. Epub 2020 Apr 22. Pneumologie. 2020. PMID: 32323287 Free PMC article. German.
-
Limitations of the ARDS criteria during high-flow oxygen or non-invasive ventilation: evidence from critically ill COVID-19 patients.Crit Care. 2022 Mar 7;26(1):55. doi: 10.1186/s13054-022-03933-1. Crit Care. 2022. PMID: 35255949 Free PMC article.
-
Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome.Clin Respir J. 2021 Jul;15(7):779-787. doi: 10.1111/crj.13361. Epub 2021 Mar 23. Clin Respir J. 2021. PMID: 33728822 Free PMC article.
-
Characteristics, outcomes and global trends of respiratory support in patients hospitalized with COVID-19 pneumonia: a scoping review.Minerva Anestesiol. 2021 Aug;87(8):915-926. doi: 10.23736/S0375-9393.21.15486-0. Epub 2021 May 26. Minerva Anestesiol. 2021. PMID: 34036769
Cited by
-
Long-Term Prognosis among COVID-19 Patients: The Predictive Role Played by Hyperinflammation and Arrhythmic Disorders in Fatal Outcome.J Clin Med. 2023 Aug 31;12(17):5691. doi: 10.3390/jcm12175691. J Clin Med. 2023. PMID: 37685758 Free PMC article.
-
Predictors of high-flow nasal cannula failure in COVID-19 patients in a northern Peruvian hospital.BMC Pulm Med. 2024 Aug 28;24(1):414. doi: 10.1186/s12890-024-03241-0. BMC Pulm Med. 2024. PMID: 39198776 Free PMC article.
-
The Use of the Modified Brixia Score for Predicting Mortality and Acute Respiratory Distress Syndrome in Patients with COVID-19 Pneumonia: What Have We Learned?Diagnostics (Basel). 2025 Jun 1;15(11):1409. doi: 10.3390/diagnostics15111409. Diagnostics (Basel). 2025. PMID: 40506981 Free PMC article.
-
The COVID-19 Driving Force: How It Shaped the Evidence of Non-Invasive Respiratory Support.J Clin Med. 2023 May 16;12(10):3486. doi: 10.3390/jcm12103486. J Clin Med. 2023. PMID: 37240592 Free PMC article. Review.
-
Factors associated with the survival of adults with COVID-19 using a high-flow nasal cannula in a tertiary hospital in northern Peru during the second wave of the pandemic.PLoS One. 2025 Apr 16;20(4):e0309855. doi: 10.1371/journal.pone.0309855. eCollection 2025. PLoS One. 2025. PMID: 40238780 Free PMC article.
References
-
- Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, COVID-ICU Gemelli Study Group et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. - DOI - PMC - PubMed
-
- Bertaina M, Nuñez-Gil IJ, Franchin L, Fernández Rozas I, Arroyo-Espliguero R, Viana-Llamas MC, HOPE COVID-19 investigators et al. Non-invasive ventilation for SARS-CoV-2 acute respiratory failure: a subanalysis from the HOPE COVID-19 registry. Emerg Med J. 2021;38:359–365. doi: 10.1136/emermed-2020-210411. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

