Mucosal-associated invariant T cells for cancer immunotherapy
- PMID: 36463401
- PMCID: PMC10014234
- DOI: 10.1016/j.ymthe.2022.11.019
Mucosal-associated invariant T cells for cancer immunotherapy
Abstract
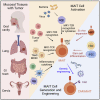
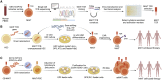
Human mucosal-associated invariant T (MAIT) cells are characterized by their expression of an invariant TCR α chain Vα7.2-Jα33/Jα20/Jα12 paired with a restricted TCR β chain. MAIT cells recognize microbial peptides presented by the highly conserved MHC class I-like molecule MR1 and bridge the innate and acquired immune systems to mediate augmented immune responses. Upon activation, MAIT cells rapidly proliferate, produce a variety of cytokines and cytotoxic molecules, and trigger efficient antitumor immunity. Administration of a representative MAIT cell ligand 5-OP-RU effectively activates MAIT cells and enhances their antitumor capacity. In this review, we introduce MAIT cell biology and their importance in antitumor immunity, summarize the current development of peripheral blood mononuclear cell-derived and stem cell-derived MAIT cell products for cancer treatment, and discuss the potential of genetic engineering of MAIT cells for off-the-shelf cancer immunotherapy.
Keywords: CAR engineering; GvHD; MAIT cell; allogeneic cell therapy; cancer immunotherapy; chimeric antigen receptor engineering; combination therapy; graft-versus-host disease; mucosal-associated invariant T cell; off-the-shelf; stem cell engineering.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.-R.L. and L.Y. are inventors on patents relating to this article filed by UCLA. L.Y. is a scientific adviser to AlzChem and Amberstone Biosciences, and a co-founder, stockholder, and advisory board member of Appia Bio. None of the declared companies contributed to or directed any of the research reported in this article.
Figures


References
-
- Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. - DOI - PubMed
-
- Tilloy F., Treiner E., Park S.H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

