A hemifused complex is the hub in a network of pathways to membrane fusion
- PMID: 36463406
- PMCID: PMC9892611
- DOI: 10.1016/j.bpj.2022.12.003
A hemifused complex is the hub in a network of pathways to membrane fusion
Abstract
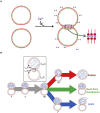
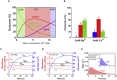
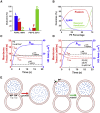
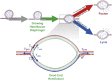
Membrane fusion is a critical step for many essential processes, from neurotransmission to fertilization. For over 40 years, protein-free fusion driven by calcium or other cationic species has provided a simplified model of biological fusion, but the mechanisms remain poorly understood. Cation-mediated membrane fusion and permeation are essential in their own right to drug delivery strategies based on cell-penetrating peptides or cation-bearing lipid nanoparticles. Experimental studies suggest calcium drives anionic membranes to a hemifused intermediate that constitutes a hub in a network of pathways, but the pathway selection mechanism is unknown. Here we develop a mathematical model that identifies the network hub as a highly dynamic hemifusion complex. Multivalent cations drive expansion of this high-tension hemifusion interface between interacting vesicles during a brief transient. The fate of this interface determines the outcome, either fusion, dead-end hemifusion, or vesicle lysis. The model reproduces the unexplained finding that calcium-driven fusion of vesicles with planar membranes typically stalls at hemifusion, and we show the equilibrated hemifused state is a novel lens-shaped complex. Thus, membrane fusion kinetics follow a stochastic trajectory within a network of pathways, with outcome weightings set by a hemifused complex intermediate.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Ginsberg L. Does Ca2+ cause fusion or lysis of unilamellar lipid vesicles? Nature. 1978;275:758–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

