Sounding the alarmins-The role of alarmin cytokines in asthma
- PMID: 36463491
- PMCID: PMC10108333
- DOI: 10.1111/all.15609
Sounding the alarmins-The role of alarmin cytokines in asthma
Abstract
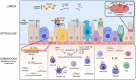
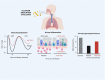
The alarmin cytokines thymic stromal lymphopoietin (TSLP), interleukin (IL)-33, and IL-25 are epithelial cell-derived mediators that contribute to the pathobiology and pathophysiology of asthma. Released from airway epithelial cells exposed to environmental triggers, the alarmins drive airway inflammation through the release of predominantly T2 cytokines from multiple effector cells. The upstream positioning of the alarmins is an attractive pharmacological target to block multiple T2 pathways important in asthma. Blocking the function of TSLP inhibits allergen-induced responses including bronchoconstriction, airway hyperresponsiveness, and inflammation, and subsequent clinical trials of an anti-TSLP monoclonal antibody, tezepelumab, in asthma patients demonstrated improvements in lung function, airway responsiveness, inflammation, and importantly, a reduction in the rate of exacerbations. Notably, these improvements were observed in patients with T2-high and with T2-low asthma. Clinical trials blocking IL-33 and its receptor ST2 have also shown improvements in lung function and exacerbation rates; however, the impact of blocking the IL-33/ST2 axis in T2-high versus T2-low asthma is unclear. To date, there is no evidence that IL-25 blockade is beneficial in asthma. Despite the considerable overlap in the cellular functions of IL-25, IL-33, and TSLP, they appear to have distinct roles in the immunopathology of asthma.
Keywords: alarmin cytokines; asthma; clinical trials; eosinophilia; exacerbation.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest related to this manuscript.
Outside of this work, GMG reports receiving consulting or speaker fees from AstraZeneca. Sanofi‐Regeneron and research grants from Biohaven, Genentech, BioGaia, Novartis; CB reports receiving consulting or speaker fees from Valeo, AstraZeneca, GSK, Grifols, Takeda, and research grants (clinical trials paid to Vancouver Coastal Health Research Institute/University of British Columbia) from Biohaven, AstraZeneca, Regeneron, GSK, Novartis, Sanofi, Teva; LPB reports receiving consulting or speaker fees from AstraZeneca, Covis, Cipla, GlaxoSmithKline, Novartis, Merck, Sanofi‐Regeneron, and research grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis, Sanofi‐Regeneron, Biohaven; DWC reports receiving research grants from Department of Medicine University of Saskatchewan, AstraZeneca, Biohaven, Novartis, CSACI, and AllerGen NCE; AC reports receiving consulting or speaker fees from AstraZeneca, Covis, Valeo pharma, GlaxoSmithKline, Sanofi‐Regeneron, and research grants from GlaxoSmithKline; RL reports receiving consulting or speaker fees from Novartis, AstraZeneca, GlaxoSmithKline, Sanofi Genzyme, and Valeo Pharma Inc research grants from Biohaven; POB reports personal fees for consulting or speaker fees from AstraZeneca, GSK, Medimmune, Chiesi, Menarini, and Covis and research grants from AstraZeneca, Medimmune, Biohaven, Merck, and Bayer. RL reports receiving consulting or speaker fees from AstraZeneca, GlaxoSmithKline, Regeneron, Sanofi Genzyme and Valeo Pharma Inc. RS reports speaker fees from AstraZeneca, Teva, Genentech and Grants‐In Aid from AstraZeneca, GlaxoSmithKline, Teva, Genentech.
Figures



References
-
- Liew FY, Girard JP, Turnquist HR. Interleukin‐33 in health and disease. Nat Immunol. 2016;16(11):676‐689. - PubMed

