Portable high-throughput multimodal immunoassay platform for rapid on-site COVID-19 diagnostics
- PMID: 36464448
- PMCID: PMC9671405
- DOI: 10.1016/j.aca.2022.340634
Portable high-throughput multimodal immunoassay platform for rapid on-site COVID-19 diagnostics
Abstract
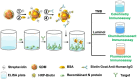
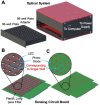
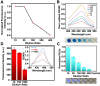
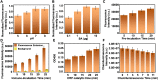
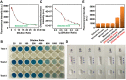
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a causal agent of Coronavirus Disease 2019 (COVID-19) has led to the global pandemic. Though the real-time reverse transcription polymerase chain reaction (RT-PCR) acting as a gold-standard method has been widely used for COVID-19 diagnostics, it can hardly support rapid on-site applications or monitor the stage of disease development as well as to identify the infection and immune status of rehabilitation patients. To suit rapid on-site COVID-19 diagnostics under various application scenarios with an all-in-one device and simple detection reagents, we propose a high-throughput multimodal immunoassay platform with fluorescent, colorimetric, and chemiluminescent immunoassays on the same portable device and a multimodal reporter probe using quantum dot (QD) microspheres modified with horseradish peroxidase (HRP) coupled with goat anti-human IgG. The recombinant nucleocapsid protein fixed on a 96-well plate works as the capture probe. In the condition with the target under detection, both reporter and capture probes can be bound by such target. When illuminated by excitation light, fluorescence signals from QD microspheres can be collected for target quantification often at a fast speed. Additionally, when pursuing simple detection without using any sensing devices, HRP-catalyzed TMB colorimetric immunoassay is employed; and when pursuing highly sensitive detection, HRP-catalyzed luminol chemiluminescent immunoassay is established. Verified by the anti-SARS-CoV-2 N humanized antibody, the sensitivities of colorimetric, fluorescent, and chemiluminescent immunoassays are respectively 20, 80, and 640 times more sensitive than that of the lateral flow colloidal gold immunoassay strip. Additionally, such a platform can simultaneously detect multiple samples at the same time thus supporting high-throughput sensing; and all these detecting operations can be implemented on-site within 50 min relying on field-operable processing and field-portable devices. Such a high-throughput multimodal immunoassay platform can provide a new all-in-one solution for rapid on-site diagnostics of COVID-19 for different detecting purposes.
Keywords: COVID-19; Chemiluminescent immunoassay; Colorimetric immunoassay; Fluorescent immunoassay; High-throughput multimodal immunoassay platform.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Development, performance evaluation, and clinical application of a Rapid SARS-CoV-2 IgM and IgG Test Kit based on automated fluorescence immunoassay.J Med Virol. 2021 May;93(5):2838-2847. doi: 10.1002/jmv.26696. Epub 2021 Mar 1. J Med Virol. 2021. PMID: 33231312 Free PMC article.
-
One-pot synthesized Au@Pt nanostars-based lateral flow immunoassay for colorimetric and photothermal dual-mode detection of SARS-CoV-2 nucleocapsid antibody.Anal Chim Acta. 2024 Mar 1;1292:342241. doi: 10.1016/j.aca.2024.342241. Epub 2024 Jan 11. Anal Chim Acta. 2024. PMID: 38309851
-
Development of a smartphone-based quantum dot lateral flow immunoassay strip for ultrasensitive detection of anti-SARS-CoV-2 IgG and neutralizing antibodies.Int J Infect Dis. 2022 Aug;121:58-65. doi: 10.1016/j.ijid.2022.04.042. Epub 2022 Apr 26. Int J Infect Dis. 2022. PMID: 35483554 Free PMC article.
-
Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19.Biosensors (Basel). 2023 Aug 3;13(8):786. doi: 10.3390/bios13080786. Biosensors (Basel). 2023. PMID: 37622872 Free PMC article. Review.
-
Molecular detections of coronavirus: current and emerging methodologies.Expert Rev Anti Infect Ther. 2022 Feb;20(2):199-210. doi: 10.1080/14787210.2021.1949986. Epub 2021 Jul 21. Expert Rev Anti Infect Ther. 2022. PMID: 34225540 Review.
Cited by
-
Overview of the Design and Application of Dual-Signal Immunoassays.Molecules. 2024 Sep 25;29(19):4551. doi: 10.3390/molecules29194551. Molecules. 2024. PMID: 39407482 Free PMC article. Review.
-
Fast and Sensitive Detection of Anti-SARS-CoV-2 IgG Using SiO2@Au@CDs Nanoparticle-Based Lateral Flow Immunoassay Strip Coupled with Miniaturized Fluorimeter.Biomolecules. 2024 Dec 9;14(12):1568. doi: 10.3390/biom14121568. Biomolecules. 2024. PMID: 39766275 Free PMC article.
-
Updates on the Biofunctionalization of Gold Nanoparticles for the Rapid and Sensitive Multiplatform Diagnosis of SARS-CoV-2 Virus and Its Proteins: From Computational Models to Validation in Human Samples.Int J Mol Sci. 2023 May 25;24(11):9249. doi: 10.3390/ijms24119249. Int J Mol Sci. 2023. PMID: 37298201 Free PMC article. Review.
References
-
- Ochani R., Asad A., Yasmin F., Shaikh S., Khalid H., Batra S., Sohail M.R., Mahmood S.F., Ochani R., Hussham A.M., Kumar A., Surani S. COVID-19 pandemic: from origins to outcomes. a comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infezioni Med. Le. 2021;29:20–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

