Esr1 but Not CYP19A1 Overexpression in Mammary Epithelial Cells during Reproductive Senescence Induces Pregnancy-Like Proliferative Mammary Disease Responsive to Anti-Hormonals
- PMID: 36464512
- PMCID: PMC9768685
- DOI: 10.1016/j.ajpath.2022.09.007
Esr1 but Not CYP19A1 Overexpression in Mammary Epithelial Cells during Reproductive Senescence Induces Pregnancy-Like Proliferative Mammary Disease Responsive to Anti-Hormonals
Abstract
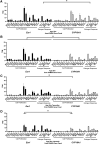
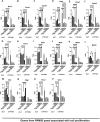
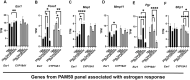
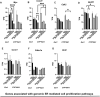
Molecular-level analyses of breast carcinogenesis benefit from vivo disease models. Estrogen receptor 1 (Esr1) and cytochrome P450 family 19 subfamily A member 1 (CYP19A1) overexpression targeted to mammary epithelial cells in genetically engineered mouse models induces largely similar rates of proliferative mammary disease in prereproductive senescent mice. Herein, with natural reproductive senescence, Esr1 overexpression compared with CYP19A1 overexpression resulted in significantly higher rates of preneoplasia and cancer. Before reproductive senescence, Esr1, but not CYP19A1, overexpressing mice are tamoxifen resistant. However, during reproductive senescence, Esr1 mice exhibited responsiveness. Both Esr1 and CYP19A1 are responsive to letrozole before and after reproductive senescence. Gene Set Enrichment Analyses of RNA-sequencing data sets showed that higher disease rates in Esr1 mice were accompanied by significantly higher expression of cell proliferation genes, including members of prognostic platforms for women with early-stage hormone receptor-positive disease. Tamoxifen and letrozole exposure induced down-regulation of these genes and resolved differences between the two models. Both Esr1 and CYP19A1 overexpression induced abnormal developmental patterns of pregnancy-like gene expression. This resolved with progression through reproductive senescence in CYP19A1 mice, but was more persistent in Esr1 mice, resolving only with tamoxifen and letrozole exposure. In summary, genetically engineered mouse models of Esr1 and CYP19A1 overexpression revealed a diversion of disease processes resulting from the two distinct molecular pathophysiological mammary gland-targeted intrusions into estrogen signaling during reproductive senescence.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures










Similar articles
-
Overexpression of Estrogen Receptor α in Mammary Glands of Aging Mice Is Associated with a Proliferative Risk Signature and Generation of Estrogen Receptor α-Positive Mammary Adenocarcinomas.Am J Pathol. 2023 Jan;193(1):103-120. doi: 10.1016/j.ajpath.2022.09.008. Epub 2022 Dec 1. Am J Pathol. 2023. PMID: 36464513 Free PMC article.
-
Comparison of tamoxifen and letrozole response in mammary preneoplasia of ER and aromatase overexpressing mice defines an immune-associated gene signature linked to tamoxifen resistance.Carcinogenesis. 2015 Jan;36(1):122-32. doi: 10.1093/carcin/bgu237. Epub 2014 Nov 23. Carcinogenesis. 2015. PMID: 25421723 Free PMC article.
-
Tamoxifen impairs prepubertal mammary development and alters expression of estrogen receptor α (ESR1) and progesterone receptors (PGR).Domest Anim Endocrinol. 2016 Jan;54:95-105. doi: 10.1016/j.domaniend.2015.10.002. Epub 2015 Oct 21. Domest Anim Endocrinol. 2016. PMID: 26619291
-
Congenital disorders of estrogen biosynthesis and action.Best Pract Res Clin Endocrinol Metab. 2022 Jan;36(1):101580. doi: 10.1016/j.beem.2021.101580. Epub 2021 Sep 13. Best Pract Res Clin Endocrinol Metab. 2022. PMID: 34538723 Review.
-
Estrogen metabolites and breast cancer.Steroids. 2015 Jul;99(Pt A):61-6. doi: 10.1016/j.steroids.2014.08.003. Epub 2014 Aug 26. Steroids. 2015. PMID: 25168343 Review.
Cited by
-
Network Pharmacology and Experimental Validation to Elucidate the Pharmacological Mechanisms of Luteolin Against Chondrocyte Senescence.Comb Chem High Throughput Screen. 2025;28(2):291-305. doi: 10.2174/0113862073273675231114112804. Comb Chem High Throughput Screen. 2025. PMID: 38299289
-
Network pharmacology and molecular docking to elucidate the mechanism of antiaging of Platycodon grandiflorus.Medicine (Baltimore). 2025 Jun 13;104(24):e42347. doi: 10.1097/MD.0000000000042347. Medicine (Baltimore). 2025. PMID: 40527795 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

