Trends in Lipid-Lowering Prescriptions: Increasing Use of Guideline-Concordant Pharmacotherapies, U.S., 2017‒2022
- PMID: 36464556
- PMCID: PMC10033441
- DOI: 10.1016/j.amepre.2022.10.010
Trends in Lipid-Lowering Prescriptions: Increasing Use of Guideline-Concordant Pharmacotherapies, U.S., 2017‒2022
Erratum in
-
Erratum to: Trends in Lipid-Lowering Prescriptions: Increasing Use of Guideline-Concordant Pharmacotherapies, U.S., 2017-2022. Sekkarie A, Park S, Therrien NL, et al. Am J Prev Med. 2023;64(4):561-566.Am J Prev Med. 2023 Aug;65(2):345. doi: 10.1016/j.amepre.2023.05.017. Epub 2023 May 29. Am J Prev Med. 2023. PMID: 37256237 No abstract available.
Abstract
Introduction: Almost one third of U.S. adults have elevated low-density lipoprotein cholesterol, increasing their risk of atherosclerotic cardiovascular disease. The 2018 American College of Cardiology/American Heart Association Multisociety Cholesterol Management Guideline recommends maximally tolerated statin for those at increased atherosclerotic cardiovascular disease risk and add-on therapies (ezetimibe and PCSK9 inhibitors) in those at very high risk and low-density lipoprotein cholesterol ≥70 mg/dL. Prescription fill trends are unknown.
Methods: Using national outpatient retail prescription data from the first quarter of 2017 to the first quarter of 2022, authors determined counts of patients who filled low-, moderate-, or high-intensity statins alone and with add-on therapies. The overall percentage change and joinpoint regression were used to assess trends. Analyses were conducted in March 2022-May 2022.
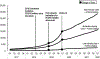
Results: During the first quarter of 2017 to the first quarter of 2022, patients filling a statin increased by 25.0%, with the greatest increase in high-intensity statins (64.1%, range=6.6-10.9 million). Low-intensity statins decreased by 29.2% (range=3.3-2.4 million). Concurrent fills of high-intensity statin and ezetimibe rose by 210% to 579,012 patients by the first quarter of 2022, with an increase in slope by the first quarter of 2019 for all statin intensities (p<0.01). Concurrent fills of a statin and PCSK9 inhibitor increased to 2,629, 16,169, and 28,651 by the first quarter of 2022 for low-, moderate-, and high-intensity statins, respectively. For patients on all statin intensities and PCSK9 inhibitor, there were statistically significant increases in slope in the second quarter of 2019 and decreases in the first quarter of 2020.
Conclusions: Patients filling moderate- and high-intensity statins and add-on ezetimibe and PCSK9 inhibitors have increased, indicating uptake of guideline-concordant lipid-lowering therapies. Improvements in the initiation and continuity of these therapies are important for atherosclerotic cardiovascular disease prevention.
Published by Elsevier Inc.
Conflict of interest statement
The authors report no conflicts of interest or financial disclosures.
Figures




References
-
- American Heart Association; American Stroke Association. Cardiovascular Disease: A Costly Burden for America - Projections through 2035. Accessed May 13, 2022. https://www.heart.org/-/media/files/get-involved/advocacy/burden-report-....
-
- Food and Drug Administration. PRALUENTTM (alirocumab) injection, & REPATHA (evolocumab) injection, for subcutaneous use: initial U.S. Approval. Accessed May 13, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125559s019s020...; https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125522s29s31lb....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

