Health-related quality-of-life analyses from a multicenter, randomized, double-blind phase 2 study of patients with differentiated thyroid cancer treated with lenvatinib 18 or 24 mg/day
- PMID: 36464853
- PMCID: PMC9972135
- DOI: 10.1002/cam4.5308
Health-related quality-of-life analyses from a multicenter, randomized, double-blind phase 2 study of patients with differentiated thyroid cancer treated with lenvatinib 18 or 24 mg/day
Abstract
Background: In the phase 2 double-blind Study 211, a starting dose of lenvatinib 18 mg/day was compared with the approved starting dose of 24 mg/day in patients with radioiodine-refractory differentiated thyroid cancer (RR-DTC). Predefined criteria for noninferiority for efficacy in the 18 mg arm were not met; safety was similar in both arms. Impact of lenvatinib treatment on health-related quality-of-life (HRQoL) was a secondary endpoint of Study 211.
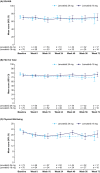
Methods: Patients with RR-DTC were randomly assigned to a blinded starting dose of lenvatinib 18 mg/day or 24 mg/day. HRQoL was assessed at baseline, every 8 weeks until Week 24, then every 16 weeks, and at the off-treatment visit, using the EQ-5D-3L and FACT-G instruments. Completion and compliance rates, mean change from baseline, and times to first and definitive deterioration were evaluated.
Results: Baseline EQ-5D and FACT-G scores, and overall changes from baseline, were comparable between patients in the lenvatinib 18 mg/day (n = 77) and 24 mg/day arms (n = 75). For the 18 mg versus 24 mg arms, least squares mean differences were -0.42 (95% CI -4.88, 4.03) for EQ-5D-VAS and 0.47 (95% CI -3.45, 4.39) for FACT-G total. Time to first deterioration did not significantly favor either arm; EQ-5D-VAS HR [18 mg/24 mg] 0.93 (95% CI 0.61-1.40), EQ-5D-HUI HR [18 mg/24 mg] 0.68 (95% CI 0.44-1.05), FACT-G total HR [18 mg/24 mg] 0.73 (95% CI 0.48-1.12). Time to definitive deterioration did not significantly favor either arm, though EQ-5D-VAS showed a trend in favor of the 24 mg arm (HR [18 mg/24 mg] 1.72; 95% CI 0.99-3.01); EQ-5D-HUI HR [18 mg/24 mg] was 0.96 (95% CI 0.57-1.63), FACT-G total HR [18 mg/24 mg] was 0.72 (95% CI 0.43-1.21).
Conclusions: In Study 211, HRQoL for patients in the lenvatinib 18 mg/day arm was not statistically different from that of patients in the 24 mg/day arm. These data further support the use of the approved lenvatinib starting dose of 24 mg/day in patients with RR-DTC.
Gov number: NCT02702388.
Keywords: HRQoL; dose intensity; kinase inhibitor; lenvatinib; quality of life; radioiodine-refractory differentiated thyroid cancer.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Matthew H. Taylor: Consulting/advisory board member (honoraria paid to MHT): Bristol Myers Squibb, Eisai Inc., Novartis, Merck, Pfizer, Bayer, Sanofi/Genzyme, Regeneron, Bayer, Array Biopharma, LOXO Oncology, Blueprint Medicines, Immuneonc, Exelixis, Cascade Prodrug. Speakers' bureau (honoraria paid to MHT): Bristol Myers Squibb, Eisai Inc., Blueprint Medicines, Merck. Research Funding (all funding to MHT's institution): Bristol Myers Squibb, Merck Sharp & Dohme Corp., Pharmacyclics, AstraZeneca, Eisai, Incyte, EMD Serono, Novartis, Seattle Genetics, AbbVie, Genentech, Eli Lilly, Roche, Acerta Pharma, Genzyme Corporation, Pfizer.
Sophie Leboulleux: Advisory Board: Bayer, Eisai, Lilly; Invited Speaker: Eisai.
Yury Panaseykin: Nothing to disclose.
Bhavana Konda: Research funding (all funding to institution): Eisai, Merck, Bristol Myers Squibb, Xencor, Eli Lilly & Co.
Christelle de La Fouchardiere: Honoraria: Eisai, Roche, Servier, Amgen, Bayer, Pierre Fabre Oncologie, Bristol Myers Squibb; Non‐financial support: Roche, Servier, Amgen, Bayer, Pierre Fabre Oncologie, Bristol Myers Squibb.
Brett G.M. Hughes: Advisory Board: MSD, BMS, Roche, Pfizer, AZ, Eisai, Takeda, Sanofi; Grant (Institutional): Amgen.
Andrew G. Gianoukakis: Advisory Board: Eisai, Exelixis.
Young Joo Park: Consultant (Steering committee): Novartis; Research funding: Lilly.
Ilia Romanov: Honoraria: Eisai, Bristol Myers Squibb, Merck Serono.
Monika K. Krzyzanowska: Advisory Board: Eisai, Ipsen, Novartis; Research funding: Ipsen, Eisai.
Diana Garbinsky is a full‐time employee of RTI Health Solutions.
Bintu Sherif is a full‐time employee of RTI Health Solutions.
Jie Janice Pan is an employee of Eisai Inc.
Terri A. Binder is a former employee of, and current consultant to, Eisai Inc., and an Amgen Inc. shareholder.
Nicholas Sauter is an employee of Eisai Inc.
Ran Xie is an employee of Eisai Inc.
Marcia S. Brose: Consulting/advisory board member (honoraria paid to MSB): Eisai Inc, Novartis, Bayer, Sanofi/Genzyme, LOXO Oncology, Blueprint Medicines, Exelixis; Research Funding (all funding to MSB's institution): Eisai, Novartis, Genentech, Eli Lilly, Roche.
Figures


References
-
- Applewhite MK, James BC, Kaplan SP, et al. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg. 2016;40(3):551‐561. - PubMed
-
- Baker F, Haffer SC, Denniston M. Health‐related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97(3):674‐681. - PubMed
-
- Müller V, Nabieva N, Häberle L, et al. Impact of disease progression on health‐related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast. 2018;37:154‐160. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

