3D-Printed Microarray Patches for Transdermal Applications
- PMID: 36465529
- PMCID: PMC9709783
- DOI: 10.1021/jacsau.2c00432
3D-Printed Microarray Patches for Transdermal Applications
Abstract
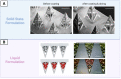
The intradermal (ID) space has been actively explored as a means for drug delivery and diagnostics that is minimally invasive. Microneedles or microneedle patches or microarray patches (MAPs) are comprised of a series of micrometer-sized projections that can painlessly puncture the skin and access the epidermal/dermal layer. MAPs have failed to reach their full potential because many of these platforms rely on dated lithographic manufacturing processes or molding processes that are not easily scalable and hinder innovative designs of MAP geometries that can be achieved. The DeSimone Laboratory has recently developed a high-resolution continuous liquid interface production (CLIP) 3D printing technology. This 3D printer uses light and oxygen to enable a continuous, noncontact polymerization dead zone at the build surface, allowing for rapid production of MAPs with precise and tunable geometries. Using this tool, we are now able to produce new classes of lattice MAPs (L-MAPs) and dynamic MAPs (D-MAPs) that can deliver both solid state and liquid cargos and are also capable of sampling interstitial fluid. Herein, we will explore how additive manufacturing can revolutionize MAP development and open new doors for minimally invasive drug delivery and diagnostic platforms.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Joseph M DeSimone reports financial support to our research group was provided by Wellcome Leap Fund and startup funds provided by Stanford University School of Medicine and the School of Engineering. Netra U. Rajesh reports financial support was provided by the Knight-Hennessy Scholarship. Joseph M DeSimone reports a relationship with Carbon that includes board membership and cofounder equity. Provisional patents have also been filed by Stanford for both the L-MAP and D-MAP technologies disclosed in this paper.
Figures








References
-
- Kolarsick P. A. J.; Kolarsick M. A.; Goodwin C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses Assoc. 2011, 3 (4), 203–213. 10.1097/JDN.0b013e3182274a98. - DOI
-
- Wei J. C. J.; Edwards G. A.; Martin D. J.; Huang H.; Crichton M. L.; Kendall M. A. F. Allometric Scaling of Skin Thickness, Elasticity, Viscoelasticity to Mass for Micro-Medical Device Translation: From Mice, Rats, Rabbits, Pigs to Humans. Sci. Rep. 2017, 7 (1), 15885.10.1038/s41598-017-15830-7. - DOI - PMC - PubMed
-
- Administering MMR Vaccine, CDC. https://www.cdc.gov/vaccines/vpd/mmr/hcp/administering-mmr.html (accessed 2022-06-20).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
