Intracellular VHHs to monitor and modulate GPCR signaling
- PMID: 36465650
- PMCID: PMC9708903
- DOI: 10.3389/fendo.2022.1048601
Intracellular VHHs to monitor and modulate GPCR signaling
Abstract
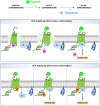
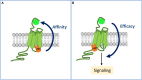
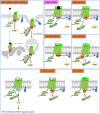
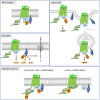
Single-domain antibody fragments, also known as VHHs or nanobodies, have opened promising avenues in therapeutics and in exploration of intracellular processes. Because of their unique structural properties, they can reach cryptic regions in their cognate antigen. Intracellular VHHs/antibodies primarily directed against cytosolic proteins or transcription factors have been described. In contrast, few of them target membrane proteins and even less recognize G protein-coupled receptors. These receptors are major therapeutic targets, which reflects their involvement in a plethora of physiological responses. Hence, they elicit a tremendous interest in the scientific community and in the industry. Comprehension of their pharmacology has been obscured by their conformational complexity, that has precluded deciphering their structural properties until the early 2010's. To that respect, intracellular VHHs have been instrumental in stabilizing G protein-coupled receptors in active conformations in order to solve their structure, possibly bound to their primary transducers, G proteins or β-arrestins. In contrast, the modulatory properties of VHHs recognizing the intracellular regions of G protein-coupled receptors on the induced signaling network have been poorly studied. In this review, we will present the advances that the intracellular VHHs have permitted in the field of GPCR signaling and trafficking. We will also discuss the methodological hurdles that linger the discovery of modulatory intracellular VHHs directed against GPCRs, as well as the opportunities they open in drug discovery.
Keywords: Cell signaling; G protein-coupled receptor; G proteins; biosensor; conformations; intracellular VHHs; β-arrestins.
Copyright © 2022 Raynaud, Gauthier, Jugnarain, Jean-Alphonse, Reiter, Bruneau and Crépieux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Figures


Comment in
-
A single-domain intrabody targeting the follicle-stimulating hormone receptor impacts FSH-induced G protein-dependent signalling.FEBS Lett. 2024 Jan;598(2):220-232. doi: 10.1002/1873-3468.14765. Epub 2023 Nov 3. FEBS Lett. 2024. PMID: 37923554
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

