Microwell culture platform maintains viability and mass of human pancreatic islets
- PMID: 36465665
- PMCID: PMC9712283
- DOI: 10.3389/fendo.2022.1015063
Microwell culture platform maintains viability and mass of human pancreatic islets
Abstract
Background: Transplantation of the human pancreatic islets is a promising approach for specific types of diabetes to improve glycemic control. Although effective, there are several issues that limit the clinical expansion of this treatment, including difficulty in maintaining the quality and quantity of isolated human islets prior to transplantation. During the culture, we frequently observe the multiple islets fusing together into large constructs, in which hypoxia-induced cell damage significantly reduces their viability and mass. In this study, we introduce the microwell platform optimized for the human islets to prevent unsolicited fusion, thus maintaining their viability and mass in long-term cultures.
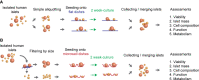
Method: Human islets are heterogeneous in size; therefore, two different-sized microwells were prepared in a 35 mm-dish format: 140 µm × 300 µm-microwells for <160 µm-islets and 200 µm × 370 µm-microwells for >160 µm-islets. Human islets (2,000 islet equivalent) were filtered through a 160 µm-mesh to prepare two size categories for subsequent two week-cultures in each microwell dish. Conventional flat-bottomed 35 mm-dishes were used for non-filtered islets (2,000 islet equivalent/2 dishes). Post-cultured islets are collected to combine in each condition (microwells and flat) for the comparisons in viability, islet mass, morphology, function and metabolism. Islets from three donors were independently tested.
Results: The microwell platform prevented islet fusion during culture compared to conventional flat bottom dishes, which improved human islet viability and mass. Islet viability and mass on the microwells were well-maintained and comparable to those in pre-culture, while flat bottom dishes significantly reduced islet viability and mass in two weeks. Morphology assessed by histology, insulin-secreting function and metabolism by oxygen consumption did not exhibit the statistical significance among the three different conditions.
Conclusion: Microwell-bottomed dishes maintained viability and mass of human islets for two weeks, which is significantly improved when compared to the conventional flat-bottomed dishes.
Keywords: cell death; diabetes; human pancreatic islets; hypoxia; islet transplantation; long-term culture; microwells; type 1 diabetes.
Copyright © 2022 Kato, Miwa, Quijano, Medrano, Ortiz, Desantis, Omori, Wada, Tatsukoshi, Kandeel, Mullen, Ku and Komatsu.
Conflict of interest statement
This study was performed as a collaborative study between AGC Techno Glass and Arthur Riggs Diabetes & Metabolism Research Institute of City of Hope. TM, AW, and KT are the employees at AGC Techno Glass. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. . The management of type 1 diabetes in adults. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care (2021) 44(11):2589–625. doi: 10.2337/dci21-0043 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

