KDEON WK-11: A short antipseudomonal peptide with promising potential
- PMID: 36465859
- PMCID: PMC9713011
- DOI: 10.3389/fchem.2022.1000765
KDEON WK-11: A short antipseudomonal peptide with promising potential
Abstract
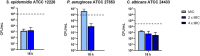
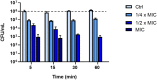
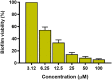
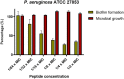
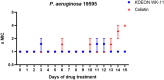
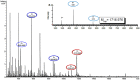
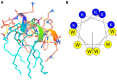
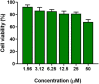
The plight of antimicrobial resistance continues to limit the availability of antibiotic treatment effective in combating resistant bacterial infections. Despite efforts made to rectify this issue and minimise its effects on both patients and the wider community, progress in this area remains minimal. Here, we de-novo designed a peptide named KDEON WK-11, building on previous work establishing effective residues and structures active in distinguished antimicrobial peptides such as lactoferrin. We assessed its antimicrobial activity against an array of bacterial strains and identified its most potent effect, against Pseudomonas aeruginosa with an MIC value of 3.12 μM, lower than its counterparts developed with similar residues and chain lengths. We then determined its anti-biofilm properties, potential mechanism of action and in vitro cytotoxicity. We identified that KDEON WK-11 had a broad range of antimicrobial activity and specific capabilities to fight Pseudomonas aeruginosa with low in vitro cytotoxicity and promising potential to express anti-lipopolysaccharide qualities, which could be exploited to expand its properties into an anti-sepsis agent.
Keywords: Pseudomonas aeruginosa; antibiofilm activity; antimicrobial peptides; lipopolysaccharides; tryptophan.
Copyright © 2022 Casciaro, Loffredo, Cappiello, O’Sullivan, Tortora, Manzer, Karmakar, Haskell, Hasan and Mangoni.
Conflict of interest statement
The authors RM, SK, AH, and SH are employed by Iuventis Technologies Inc. (DBA Immunotrex), a company developing KDEON peptide. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Andreev K., Bianchi C., Laursen J. S., Citterio L., Hein-Kristensen L., Gram L., et al. (2014). Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta - Biomembr. 1838, 2492–2502. 10.1016/j.bbamem.2014.05.022 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

