Implementing HPV testing in 9 Latin American countries: The laboratory perspective as observed in the ESTAMPA study
- PMID: 36465901
- PMCID: PMC9714610
- DOI: 10.3389/fmed.2022.1006038
Implementing HPV testing in 9 Latin American countries: The laboratory perspective as observed in the ESTAMPA study
Abstract
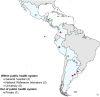
Background: Replacement of cytology screening with HPV testing is recommended and essential for cervical cancer elimination. HPV testing for primary screening was implemented in 12 laboratories within 9 Latin American countries, as part of the ESTAMPA cervical cancer screening study. Our observations provide information on critical operational aspects for HPV testing implementation in diverse resource settings.
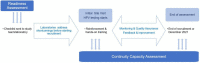
Methods: We describe the implementation process of HPV testing in ESTAMPA, focusing on laboratory aspects. We assess the readiness of 12 laboratories to start HPV testing and their continuity capacity to maintain good quality HPV testing until end of recruitment or up to December 2021. Readiness was based on a checklist. Information from the study database; regular meetings and monitoring visits; and a questionnaire on laboratory operational aspects sent in May 2020 were used to assess continuity capacity. Compliance with seven basic requirements (readiness) and eight continuity requirements (continuity capacity) was scored (1 = compliant, 0 = not compliant) and totaled to classify readiness and continuity capacity as very limited, limited, moderate or high. Experiences, challenges, and enablers of the implementation process are also described.
Results: Seven of 12 laboratories had high readiness, three moderate readiness, and of two laboratories new to HPV testing, one had limited readiness and the other very limited readiness. Two of seven laboratories with high readiness also showed high continuity capacity, one moderate continuity capacity, and the other four showed limited continuity capacity since they could not maintain good quality HPV testing over time. Among three laboratories with moderate readiness, one kept moderate continuity capacity and two reached high continuity capacity. The two laboratories new to HPV testing achieved high continuity capacity. Based on gained expertise, five laboratories have become part of national screening programs.
Conclusion: High readiness of laboratories is an essential part of effective implementation of HPV testing. However, high readiness is insufficient to guarantee HPV testing high continuity capacity, for which a "culture of quality" should be established with regular training, robust monitoring and quality assurance systems tailored to local context. All efforts to strengthen HPV laboratories are valuable and crucial to guarantee effective implementation of HPV-based cervical screening.
Keywords: ESTAMPA study; HPV testing; HPV testing implementation; Latin America; cervical cancer screening; readiness and continuity capacity.
Copyright © 2022 Rol, Picconi, Ferrera, Sánchez, Hernández, Lineros, Peraza, Brizuela, Mendoza, Mongelós, Cabrera, Rodríguez de la Peña, Correa, Terán, Colque Reynaga, García, Ramírez, Hernández-Nevarez, Doimi, Ramón, Arias-Stella, Zúñiga, Villagra, Bobadilla, Cardinal, Valls, Lucas, Baena, Fleider, Venegas, Cruz-Valdez, Rodríguez, Calderón, Wiesner, Luciani, Broutet, Herrero and Almonte.
Conflict of interest statement
Author MH was employed by SMS-Oncology, Amsterdam, Netherlands. Author FD was employed by Laboratorio de Patología Oncológica, SAC, Peru. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

