Chinese patent medicines combined with hormone replacement therapy for premature ovarian failure: A Bayesian network meta-analysis
- PMID: 36465907
- PMCID: PMC9712806
- DOI: 10.3389/fmed.2022.1043390
Chinese patent medicines combined with hormone replacement therapy for premature ovarian failure: A Bayesian network meta-analysis
Abstract
Objective: The objective of this study was to compare the efficacy differences between Chinese patent medicines combined with hormone replacement therapy (HRT) in the treatment of premature ovarian failure (POF) by the Bayesian network meta-analysis (NMA) method.
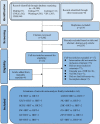
Methods: Randomized controlled trials (RCTs) reporting Chinese patent medicine combined with HRT for POF included Medline (via PubMed), Embase, Cochrane Library, China National Knowledge Infrastructure Database (CNKI), Wanfang Database (Wanfang), VIP Database (VIP), and China Biology Medicine Database (CBM) from the inception of the databases to July 2022. Two researchers independently screened the articles, extracted data, and evaluated the quality. The literature that met the inclusion criteria was screened out, the quality and risk of bias of the included studies were assessed according to the Cochrane 5.1 manual and RevMan 5.4, and NMA was performed using Stata 15.0 and R software.
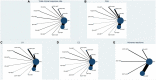
Results: Sixty-four RCTs involving 5,675 individuals containing 12 oral Chinese patent medicines combined with HRT were enrolled into the current NMA. The results showed that when compared with patients using only HRT, the total clinical response rate is greater in patients using HRT combined with one of these 12 oral Chinese patent medicines. Among them, Zuogui pills + HRT [odds ratio (OR) = 3.92; 95% credible interval (CrI) = 0.86, 23.84; SUCRA = 73.76%] is most likely to be the best intervention, and the suboptimal intervention is Guishen pills + HRT (OR = 3.22, 95% CrI = 1.16, 9.44, SUCRA = 70.60%).
Conclusion: Chinese patent medicines combined with HRT were more effective than HRT alone in the treatment of POF. Zuogui pills are good at decreasing follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and more effective in the improvement of total clinical response rate; Xuefu Zhuyu capsule is also good at decreasing FSH. Ziheche capsule is an expert in improving estradiol level; Kuntai capsule shows the lowest incidence of adverse reactions. However, the quality of the literature included in this study is relatively low, so it may affect the results of the study. Therefore, higher quality and multi-center trial would be necessary for supporting these results.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42022350587].
Keywords: Chinese patent medicine; hormone replacement therapy (HRT); network meta-analysis (NMA); premature ovarian failure (POF); validity.
Copyright © 2022 Zhong, Li, Yin, Bin, Zhou and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Efficacy and safety of Chinese patent medicines combined with conventional therapies for endometriosis: A systematic review and bayesian network meta-analysis.J Ethnopharmacol. 2025 Mar 13;343:119465. doi: 10.1016/j.jep.2025.119465. Epub 2025 Feb 8. J Ethnopharmacol. 2025. PMID: 39929398
-
A comparison of the effects of Chinese non-pharmaceutical therapies for premature ovarian failure: A PRISMA-compliant systematic review and network meta-analysis.Medicine (Baltimore). 2020 Jun 26;99(26):e20958. doi: 10.1097/MD.0000000000020958. Medicine (Baltimore). 2020. PMID: 32590807 Free PMC article.
-
Efficacy and safety of oral Chinese patent medicine for benign prostatic hyperplasia: a network meta-analysis of randomized controlled trials.Front Med (Lausanne). 2024 Dec 27;11:1483864. doi: 10.3389/fmed.2024.1483864. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39802886 Free PMC article.
-
[Network Meta-analysis of oral Chinese patent medicine in treatment of migraine].Zhongguo Zhong Yao Za Zhi. 2020 Nov;45(21):5068-5082. doi: 10.19540/j.cnki.cjcmm.20200730.503. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 33350222 Chinese.
-
Comparative efficacy of Chinese patent medicines for non-alcoholic fatty liver disease: A network meta-analysis.Front Pharmacol. 2023 Jan 4;13:1077180. doi: 10.3389/fphar.2022.1077180. eCollection 2022. Front Pharmacol. 2023. PMID: 36686656 Free PMC article.
Cited by
-
Mechanism of Zuogui pill enhancing ovarian function and skin elastic repair in premature aging rats based on artificial intelligence medical image analysis.Skin Res Technol. 2024 Sep;30(9):e70050. doi: 10.1111/srt.70050. Skin Res Technol. 2024. Retraction in: Skin Res Technol. 2024 Dec;30(12):e70156. doi: 10.1111/srt.70156. PMID: 39246259 Free PMC article. Retracted.
-
Mechanism Analysis of Zuogui and Yougui Pills on Diabetic Nephropathy Through Transcriptional Regulatory Networks of HIF1A and PPARA.Food Sci Nutr. 2025 May 23;13(6):e70317. doi: 10.1002/fsn3.70317. eCollection 2025 Jun. Food Sci Nutr. 2025. PMID: 40417738 Free PMC article.
-
Successful treatment of Heyan Kuntai capsule combined with hormonal therapy in an adolescent with diminished ovarian reserve: a case report.J Ovarian Res. 2025 Jul 3;18(1):143. doi: 10.1186/s13048-025-01716-0. J Ovarian Res. 2025. PMID: 40611296 Free PMC article.
References
-
- Tinjić S, Abazović D, Ljubić D, Vojvodić D, Božanović T, Ibrišimović M, et al. Influence of autologous in vitro activation of ovaries by stem cells and growth factors on endocrine and reproductive function of patients with ovarian insufficiency-a clinical trial study. Int J Fertil Steril. (2021) 15:178–88. 10.22074/IJFS.2020.134678 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

