Hyalocytes in proliferative vitreo-retinal diseases
- PMID: 36466118
- PMCID: PMC9718005
- DOI: 10.1080/17469899.2022.2100764
Hyalocytes in proliferative vitreo-retinal diseases
Abstract
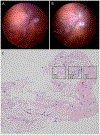
Introduction: Hyalocytes are sentinel macrophages residing within the posterior vitreous cortex anterior to the retinal inner limiting membrane (ILM). Following anomalous PVD and vitreoschisis, hyalocytes contribute to paucicellular (vitreo-macular traction syndrome, macular holes) and hypercellular (macular pucker, proliferative vitreo-retinopathy, proliferative diabetic vitreo-retinopathy) diseases.
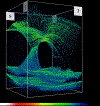
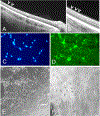
Areas covered: Studies of human tissues employing dark-field, phase, and electron microscopy; immunohistochemistry; and in vivo imaging of human hyalocytes.
Expert opinion: Hyalocytes are important in early pathophysiology, stimulating cell migration and proliferation, as well as subsequent membrane contraction and vitreo-retinal traction. Targeting hyalocytes early could mitigate advanced disease. Ultimately, eliminating the role of vitreous and hyalocytes may prevent proliferative vitreo-retinal diseases entirely.
Keywords: Vitreous; anomalous PVD; hyalocytes; macular pucker; proliferative diabetic vitreo-retinopathy; proliferative vitreo-retinopathy; vitreoschisis.
Figures













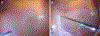
References
-
- Boneva S, Wolf J, Wieghofer P et al. Hyalocyte functions and immunology. Exp. Rev. Opthalmol. [In Press] (2022).
-
- Sebag J. Vitreous & Vitreo-Retinal Interface. In: Schachat AP, editor. Ryan’s Retina. 6th ed. Elsevier; 2018. p. 544–581.
-
- Sebag J. Vitreous and Vision Degrading Myodesopsia. Progress in Retinal and Eye Research. 2020;79:100847. - PubMed
-
- Chew L, Sebag J. Vitreous. Adler’s Physiology of the Eye. 12th ed. Philadelphia: Elsevier; 2022. p. (in press).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
