'Root of all success': Plasticity in root architecture of invasive wild radish for adaptive benefit
- PMID: 36466265
- PMCID: PMC9709435
- DOI: 10.3389/fpls.2022.1035089
'Root of all success': Plasticity in root architecture of invasive wild radish for adaptive benefit
Abstract
Successful plant establishment in a particular environment depends on the root architecture of the seedlings and the extent of edaphic resource utilization. However, diverse habitats often pose a predicament on the suitability of the fundamental root structure of a species that evolved over a long period. We hypothesized that the plasticity in the genetically controlled root architecture in variable habitats provides an adaptive advantage to worldwide-distributed wild radish (Raphanus raphanistrum, Rr) over its close relative (R. pugioniformis, Rp) that remained endemic to the East Mediterranean region. To test the hypothesis, we performed a reciprocal comparative analysis between the two species, growing in a common garden experiment on their native soils (Hamra/Sandy for Rr, Terra Rossa for Rp) and complementary controlled experiments mimicking the major soil compositions. Additionally, we analyzed the root growth kinetics via semi-automated digital profiling and compared the architecture between Rr and Rp. In both experiments, the primary roots of Rr were significantly longer, developed fewer lateral roots, and showed slower growth kinetics than Rp. Multivariate analyses of seven significant root architecture variables revealed that Rr could successfully adapt to different surrogate growth conditions by only modulating their main root length and number of lateral roots. In contrast, Rp needs to modify several other root parameters, which are very resource-intensive, to grow on non-native soil. Altogether the findings suggest an evo-devo adaptive advantage for Rr as it can potentially establish in various habitats with the minimal tweak of key root parameters, hence allocating resources for other developmental requirements.
Keywords: East Mediterranean; Raphanus; adaption; habitat preference; root plasticity; root system architecture (RSA); soil surrogates.
Copyright © 2022 Bhattacharya, Gröne, Przesdzink, Ziffer-Berger, Barazani, Mummenhoff and Kappert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


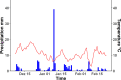

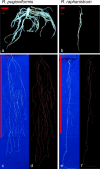
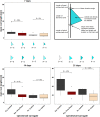
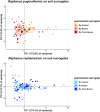
Similar articles
-
Seed dispersal of wild radishes and its association with within-population spatial distribution.BMC Ecol. 2020 May 11;20(1):30. doi: 10.1186/s12898-020-00297-4. BMC Ecol. 2020. PMID: 32393235 Free PMC article.
-
[Response of fine roots to soil nutrient spatial heterogeneity].Ying Yong Sheng Tai Xue Bao. 2004 Jun;15(6):1063-8. Ying Yong Sheng Tai Xue Bao. 2004. PMID: 15362636 Review. Chinese.
-
Genomic differentiation and SNP variation reveal local adaptations to eastern Mediterranean environmental conditions in wild radishes.Ann Bot. 2025 Mar 12:mcaf039. doi: 10.1093/aob/mcaf039. Online ahead of print. Ann Bot. 2025. PMID: 40072280
-
Jatropha curcas L. root structure and growth in diverse soils.ScientificWorldJournal. 2013 Jun 5;2013:827295. doi: 10.1155/2013/827295. Print 2013. ScientificWorldJournal. 2013. PMID: 23844412 Free PMC article.
-
Root plasticity under abiotic stress.Plant Physiol. 2021 Nov 3;187(3):1057-1070. doi: 10.1093/plphys/kiab392. Plant Physiol. 2021. PMID: 34734279 Free PMC article. Review.
Cited by
-
Comparative pericarp biomechanics and germination physiology of Raphanus raphanistrum and Raphanus pugioniformis indehiscent fruits.Ann Bot. 2025 May 9;135(5):977-990. doi: 10.1093/aob/mcaf015. Ann Bot. 2025. PMID: 39868575 Free PMC article.
References
-
- Batjes N. H., Ribeiro E., Van Oostrum A. (2020). Standardised soil profile data to support global mapping and modelling (WoSIS snapshot 2019). Earth System Sci. Data 12 (1), 299–320. doi: 10.5194/essd-12-299-2020 - DOI
-
- Cheam A. H., Code G. R. (1995). The biology of Australian weeds 24. raphanus raphanistrum l. Plant Prot. Q. 10, 2–2.
LinkOut - more resources
Full Text Sources

