Functional characterization of the sugarcane (Saccharum spp.) ammonium transporter AMT2;1 suggests a role in ammonium root-to-shoot translocation
- PMID: 36466275
- PMCID: PMC9716016
- DOI: 10.3389/fpls.2022.1039041
Functional characterization of the sugarcane (Saccharum spp.) ammonium transporter AMT2;1 suggests a role in ammonium root-to-shoot translocation
Abstract
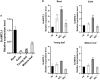
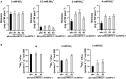
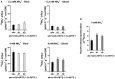
AMMONIUM TRANSPORTER/METHYLAMMONIUM PERMEASE/RHESUS (AMT) family members transport ammonium across membranes in all life domains. Plant AMTs can be categorized into AMT1 and AMT2 subfamilies. Functional studies of AMTs, particularly AMT1-type, have been conducted using model plants but little is known about the function of AMTs from crops. Sugarcane (Saccharum spp.) is a major bioenergy crop that requires heavy nitrogen fertilization but depends on a low carbon-footprint for competitive sustainability. Here, we identified and functionally characterized sugarcane ScAMT2;1 by complementing ammonium uptake-defective mutants of Saccharomyces cerevisiae and Arabidopsis thaliana. Reporter gene driven by the ScAMT2;1 promoter in A. thaliana revealed preferential expression in the shoot vasculature and root endodermis/pericycle according to nitrogen availability and source. Arabidopsis quadruple mutant plants expressing ScAMT2;1 driven by the CaMV35S promoter or by a sugarcane endogenous promoter produced significantly more biomass than mutant plants when grown in NH4 + and showed more 15N-ammonium uptake by roots and nitrogen translocation to shoots. In A. thaliana, ScAMT2;1 displayed a Km of 90.17 µM and Vmax of 338.99 µmoles h-1 g-1 root DW. Altogether, our results suggest that ScAMT2;1 is a functional high-affinity ammonium transporter that might contribute to ammonium uptake and presumably to root-to-shoot translocation under high NH4 + conditions.
Keywords: AMT2 subfamily; ammonium uptake; nitrogen use efficiency; quadruple mutant; transport kinetics; xylem loading.
Copyright © 2022 Koltun, Maniero, Vitti, de Setta, Giehl, Lima and Figueira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ariz I., Boeckstaens M., Gouveia C., Martins A. P., Sanz-Luque E., Fernández E., et al. . (2018). Nitrogen isotope signature evidences ammonium deprotonation as a common transport mechanism for the AMT-Mep-Rh protein superfamily. Sci. Adv. 4, eaar3599. doi: 10.1126/sciadv.aar3599 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

