Combined Minimally Invasive Treatment of Teeth Discoloration Caused by Occupational Exposure to Bronze Alloy
- PMID: 36466366
- PMCID: PMC9712005
- DOI: 10.1155/2022/3399120
Combined Minimally Invasive Treatment of Teeth Discoloration Caused by Occupational Exposure to Bronze Alloy
Abstract
Recently, dental bleaching has been frequently sought by patients to improve the appearance and color of the teeth. Among various treatment options, in-office bleaching is commonly preferred by patients with severely discolored teeth due to its fast aesthetic results. In addition, other pretreatment methods, such as air-powder polishing, have been reported to increase the efficacy of bleaching treatment. Compared to other causes of tooth discoloration, occupational tooth discoloration caused by bronze alloy and further treatment have not been sufficiently documented in the literature. In the present case report, we explain a case of tooth discoloration following exposure to a bronze alloy and a conservative clinical approach used for the management of tooth discoloration. A 15-year-old male patient who worked in a foundry was presented with tooth discoloration. At the first session, a rubber-cap prophylaxis was performed. After one week, air-powder polishing was used to remove the remaining stains. Finally, at the third session, two cycles of bleaching were performed using hydrogen peroxide gel 35%, each for 20 minutes. Photographs were taken at the end of each session and used for visual evaluation. The final result was aesthetically satisfactory.
Copyright © 2022 Shahram Amirifar et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding the publication of this manuscript.
Figures
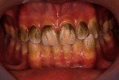

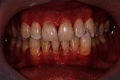
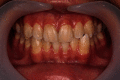
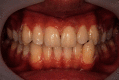
References
-
- Dayan D., Heifferman A., Gorski M., Begleiter A. Tooth discoloration--extrinsic and intrinsic factors. Quintessence International Dental Digest . 1983;14(2):195–199. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

