The role of SIRT1 in the process of Toxoplasma gondii infection of RAW 264.7 macrophages
- PMID: 36466662
- PMCID: PMC9713941
- DOI: 10.3389/fmicb.2022.1017696
The role of SIRT1 in the process of Toxoplasma gondii infection of RAW 264.7 macrophages
Abstract
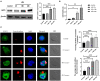
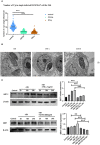
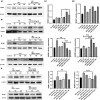
Toxoplasma gondii is an opportunistic pathogenic protozoan that can infect almost all kinds of warm-blooded animals, including humans. T. gondii can evade the host's immune response, a process known as immune evasion. Our main objective was to evaluate the role played by Sirtuin1 (SIRT1) [one of the sirtuins (SIRTs) that are a family of nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylases (HDACs)] in the T. gondii infection of RAW264.7 macrophages. In this study, we evaluated and observed alterations in the activity, expression, and localization of SIRT1 and assessed its involvement in the CD154/IFN-γ (CD40 ligand/interferon gamma) killing pathway and in autophagy during T. gondii infection. The inhibition of SIRT1 in host cells effectively reduced the number of intracellular tachyzoites, and the mechanism behind this effect might be the upregulation of IRGM1 [murine ortholog of IRGM (immunity-related GTPase family M)] and the initiation of autophagy. To the best of our knowledge, our study is the first to prove that T. gondii infection upregulates SIRT1 in RAW264.7 cells and that the inhibition of SIRT1 reduces the number of intracellular tachyzoites. Moreover, the upregulation of IRGM1 and the activation of autophagy may contribute to the intracellular inhibition of T. gondii caused by SIRT1 inhibition.
Keywords: SIRT1; Toxoplasma gondii; autophagy; innate immunity; macrophages.
Copyright © 2022 Dong, Jiang, Zhang, Qin, Chen and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

