Bacterial, fungal, and interkingdom microbiome features of exclusively breastfeeding dyads are associated with infant age, antibiotic exposure, and birth mode
- PMID: 36466688
- PMCID: PMC9714262
- DOI: 10.3389/fmicb.2022.1050574
Bacterial, fungal, and interkingdom microbiome features of exclusively breastfeeding dyads are associated with infant age, antibiotic exposure, and birth mode
Abstract
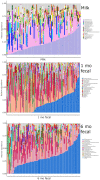
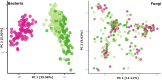
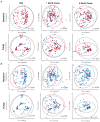
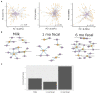
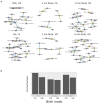
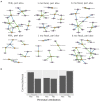
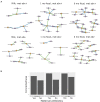
The composition and function of early life gut bacterial communities (microbiomes) have been proposed to modulate health for the long term. In addition to bacteria, fungi (mycobiomes) also colonize the early life gut and have been implicated in health disorders such as asthma and obesity. Despite the potential importance of mycobiomes in health, there has been a lack of study regarding fungi and their interkingdom interactions with bacteria during infancy. The goal of this study was to obtain a more complete understanding of microbial communities thought to be relevant for the early life programming of health. Breastmilk and infant feces were obtained from a unique cohort of healthy, exclusively breastfeeding dyads recruited as part of the Mothers and Infants Linked for Healthy Growth (MILk) study with microbial taxa characterized using amplicon-based sequencing approaches. Bacterial and fungal communities in breastmilk were both distinct from those of infant feces, consistent with niche-specific microbial community development. Nevertheless, overlap was observed among sample types (breastmilk, 1-month feces, 6-month feces) with respect to the taxa that were the most prevalent and abundant. Self-reported antibacterial antibiotic exposure was associated with micro- as well as mycobiome variation, which depended upon the subject receiving antibiotics (mother or infant), timing of exposure (prenatal, peri- or postpartum), and sample type. In addition, birth mode was associated with bacterial and fungal community variation in infant feces, but not breastmilk. Correlations between bacterial and fungal taxa abundances were identified in all sample types. For infant feces, congruency between bacterial and fungal communities was higher for older infants, consistent with the idea of co-maturation of bacterial and fungal gut communities. Interkingdom connectedness also tended to be higher in older infants. Additionally, higher interkingdom connectedness was associated with Cesarean section birth and with antibiotic exposure for microbial communities of both breastmilk and infant feces. Overall, these results implicate infant age, birth mode, and antibiotic exposure in bacterial, fungal and interkingdom relationship variation in early-life-relevant microbiomes, expanding the current literature beyond bacteria.
Keywords: bacteria; breastmilk; fungi; infant gut; interkingdom interactions; microbial community variation; microbiome; mycobiome.
Copyright © 2022 Heisel, Johnson, Gonia, Dillon, Skalla, Haapala, Jacobs, Nagel, Pierce, Fields, Demerath, Knights and Gale.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Al-Ghalith G., Knights D. (2020). github.com/ knights-lab/BURST: BURST v1.00.
-
- Aloisio I., Quagliariello A., De Fanti S., Luiselli D., De Filippo C., Albanese D., et al. . (2016). Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl. Microbiol. Biotechnol. 100, 5537–5546. doi: 10.1007/s00253-016-7410-2 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

