Evolutionary analyses of polymeric immunoglobulin receptor (pIgR) in the mammals reveals an outstanding mutation rate in the lagomorphs
- PMID: 36466819
- PMCID: PMC9716071
- DOI: 10.3389/fimmu.2022.1009387
Evolutionary analyses of polymeric immunoglobulin receptor (pIgR) in the mammals reveals an outstanding mutation rate in the lagomorphs
Abstract
Background: The transcytosis of polymeric immunoglobulins, IgA and IgM, across the epithelial barrier to the luminal side of mucosal tissues is mediated by the polymeric immunoglobulin receptor (pIgR). At the luminal side the extracellular ligand binding region of pIgR, the secretory component (SC), is cleaved and released bound to dimeric IgA (dIgA), protecting it from proteolytic degradation, or in free form, protecting the mucosa form pathogens attacks. The pIgR was first cloned for rabbit in early 1980's and since then has been described for all vertebrates, from fish to mammals. The existence of more than one functional pIgR alternative-spliced variant in the European rabbit, the complete pIgR as other mammals and a shorter pIgR lacking two SC exons, raised the question whether other lagomorphs share the same characteristics and how has the PIGR gene evolved in these mammals.
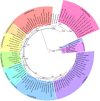
Results: To investigate these questions, we sequenced expressed pIgR genes for other leporid genus, Lepus spp., and obtained and aligned pIgR sequences from representative species of all mammalian orders. The obtained mammalian phylogeny, as well as the Bayesian inference of evolutionary rates and genetic distances, show that Lagomorpha pIgR is evolving at a higher substitution rate. Codon-based analyses of positive selection show that mammalian pIgR is evolving under strong positive selection, with strong incidence in the domains excised from the rabbit short pIgR isoform. We further confirmed that the hares also express the two rabbit pIgR isoforms.
Conclusions: The Lagomorpha pIgR unique evolutionary pattern may reflect a group specific adaptation. The pIgR evolution may be linked to the unusual expansion of IgA genes observed in lagomorphs, or to neofunctionalization in this group. Further studies are necessary to clarify the driving forces behind the unique lagomorph pIgR evolution.
Keywords: european rabbit; evolution; fc receptors; immune system; positive selection.
Copyright © 2022 Neves, de Sousa-Pereira, Melo-Ferreira, Esteves and Pinheiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fubara ES, Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol (1973) 111(2):395–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

