Hepatitis B functional cure and immune response
- PMID: 36466821
- PMCID: PMC9714500
- DOI: 10.3389/fimmu.2022.1075916
Hepatitis B functional cure and immune response
Abstract
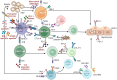
Hepatitis B virus (HBV) is a hepatotropic virus, which damage to hepatocytes is not direct, but through the immune system. HBV specific CD4+ T cells can induce HBV specific B cells and CD8+ T cells. HBV specific B cells produce antibodies to control HBV infection, while HBV specific CD8+ T cells destroy infected hepatocytes. One of the reasons for the chronicity of HBV infection is that it cannot effectively activate adoptive immunity and the function of virus specific immune cells is exhausted. Among them, virus antigens (including HBV surface antigen, e antigen, core antigen, etc.) can inhibit the function of immune cells and induce immune tolerance. Long term nucleos(t)ide analogues (NAs) treatment and inactive HBsAg carriers with low HBsAg level may "wake up" immune cells with abnormal function due to the decrease of viral antigen level in blood and liver, and the specific immune function of HBV will recover to a certain extent, thus becoming the "dominant population" for functional cure. In turn, the functional cure will further promote the recovery of HBV specific immune function, which is also the theoretical basis for complete cure of hepatitis B. In the future, the complete cure of chronic HBV infection must be the combination of three drugs: inhibiting virus replication, reducing surface antigen levels and specific immune regulation, among which specific immunotherapy is indispensable. Here we review the relationship, mechanism and clinical significance between the cure of hepatitis B and immune system.
Keywords: adaptive immunity; antiviral; chronic hepatitis B; functional cure; innate immunity.
Copyright © 2022 Zheng, Wang and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Hepatitis b (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (Accessed October 21, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

